Most pharma and MedTech companies have had digital transformation on their to-do list for quite some time. Unfortunately, only a few (roughly 20%) have actually pivoted from doing digital to being digital.
So, what differentiates these successful companies from those that are lagging behind in the way of digital transformation? This article will discuss how pharma and MedTech companies can move the needle and truly be digital.
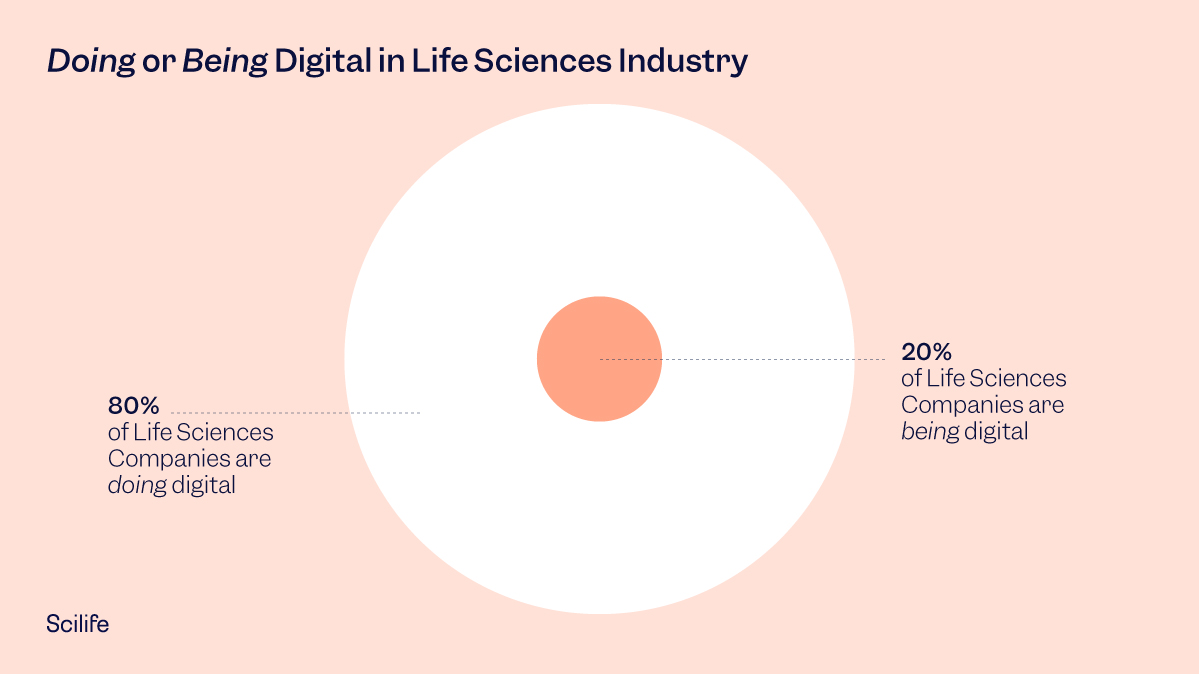 Data from Deloitte and MIT Sloan Management Review’s fourth annual study on digital maturity.
Data from Deloitte and MIT Sloan Management Review’s fourth annual study on digital maturity.
Before we begin, let’s examine the difference between doing digital and being digital.
Companies are doing digital when:
- Digital transformation has been on their goal sheet for the past few years, with no considerable success or measurable progress.
- They have a semi-digital process where part of their work is done manually, and part of their work is completed digitally.
- They have implemented limited digital processes, with most sites still using paper-based or semi-digital systems.
- They save information locally in a computerized format but depend on human resources to maintain it.
Companies are being digital when:
- Digital transformation is no longer on their goal sheet.
- They have streamlined the workflows of all business processes, with seamless digital interaction (i.e., the output from one business process automatically becomes the input for the next process).
- There is end-to-end automation.
- The system is globally-available and self-sufficient.
Vision
The first step in becoming a truly digital company is to set out a clear vision. To do so, you’ll need to answer questions such as:
- What are your company’s key business processes?
- How are these processes interrelated?
- What is the flow of the processes you want to digitalize?
- What are the pain-points of the existing processes that you don't want to see in the digitalized versions?
- Can any non-value-added activities be eliminated in the digitalized processes?
- Is your vision for digitalization eliminating manual workflows?
- What are the regulatory requirements (21 CFR 11, GAMP 5, MDR...) your company needs to meet?
- Are there any international standards your company must consider?
Once you answer the above questions, you will have a better understanding of your company’s “wants,” which you can then describe to your service provider. From there, you can move on to the next step of finding the right vendor.
Finding the Right Vendor
Failure to find the right vendor can be the final nail in your proverbial digital transformation coffin. You might have to conduct a bit of market research to sail through this problem. Accordingly, be sure to focus on the following when selecting your vendor:
- Whether the vendor has experience working with companies in the life sciences space
- Other life sciences companies’ relationship and experience working with the vendor
- The strength of the vendor’s customer success team
- How you can ask questions, raise concerns, or otherwise communicate with the vendor’s customer success team
Transformation Time
Many companies worry that complete digital transformation is time-consuming, and that making significant changes in the way of digitalization will reduce their operational efficiency.
However, with a digital transformation partner like Scilife, digital transformation times are quick and seamless. This is because Scilife provides an end-to-end digital transformation software solution, holding your hand throughout the rapid adoption process by offering additional support for data import, integration, and training with the active directories.
This is designed to ease a lot of burden your internal IT and QA teams would otherwise be facing.
Regulatory Compliance
Life sciences industries have to meet stringent regulatory compliance for Good Manufacturing Practices and Data Integrity. To this end, sourcing a digital solution that meets all compliance criteria requires a rigorous vendor qualification process.
Companies can overcome this hurdle by opting for 21 CFR Part 11 compliant software that many of their industry peers have already adopted.
Computer System Validation (CSV)
Software validation is generally a lengthy process. It is also relatively new in the life sciences industry. As a result, many companies don't have dedicated Computer System Validation teams, and so they struggle to establish their CSV processes.
However, overcoming this issue is relatively simple. Companies can easily hire external CSV consultants, including those with expansive domain experience, as well as solutions like Scilife which uses a GAMP5 validation approach for validating the system on the Amazon Web Services (AWS) platform.
.
Semi-Digital vs. Digital
Many companies misunderstand what it means to be digital. Some think it simply involves saving documents on a computer. Although this is an excellent step toward digitalization, it doesn’t represent the full picture. Instead, we might call this a semi-digital process. And this is where companies get caught in a never-ending doing digital loop.
What this means is that they are literally doing things like manually scanning documents, signing contracts, and saving digital versions of important files. Yet real digital transformation is almost the opposite of this. In an actual digital transformation process, there is no manual intervention needed. Instead, the entire process flow is digitalized.
For example, Scilife’s Document Control Module automates everything—from creating a document to getting it approved, and then linking it to all relevant files. It also auto-updates new versions of the documents, making older versions digitally obsolete when needed.
Accessibility
In recent times, we have seen a major increase in the number of remote teams. So naturally, companies are struggling to find a single platform that will work in all remote locations around the world—giving rise to partially-digitalized organizations that are digital on some sites.
However, to become 100% digital, you must look for solutions that are easy to implement across the board. A cloud-based platform can achieve this well, as the system can be implemented globally without the need for any site- or system-specific installations.
Affordability
Last but not least, it is essential to select an affordable digital transformation platform. If you want to be digital without significantly reducing your cost of operations, you’ll need to pay close attention to your budget for the initiative.
This means that to make a strong business case, you’ll want to invest in a platform that will bring a return on your investment in one to three years, and go on to widen your profit margins shortly thereafter. Startups should aim to recover the cost of their digital transformation even faster. They can achieve this by choosing pay-as-you-go and pay-per-user platforms.
Conclusion
Ultimately, championing an end-to-end digital transformation project requires careful planning, vendor selection, implementation, and execution. Carrying all the burden yourself can prolong the time it takes to go from doing digital to being digital.
That said, the process becomes much easier if you choose to partner with an experienced service provider—a proven expert with extensive experience and a satisfied customer base.
Discover how Scilife smart QMS can help you succeed in the transformation!