According to the U.S. Food and Drug Administration (FDA), data integrity is the completeness, consistency, and accuracy of data. The agency places a significant emphasis on data integrity in regulated industries such as pharmaceuticals, biotechnology, medical devices, and other areas falling under its purview. The FDA's definition of data integrity is embedded in its guidance documents, regulations, and inspections.
One of the key documents outlining the FDA's perspective on data integrity is the "Data Integrity and Compliance with CGMP" guidance, which was issued in 2016. In this guidance, the FDA defines data integrity as follows:
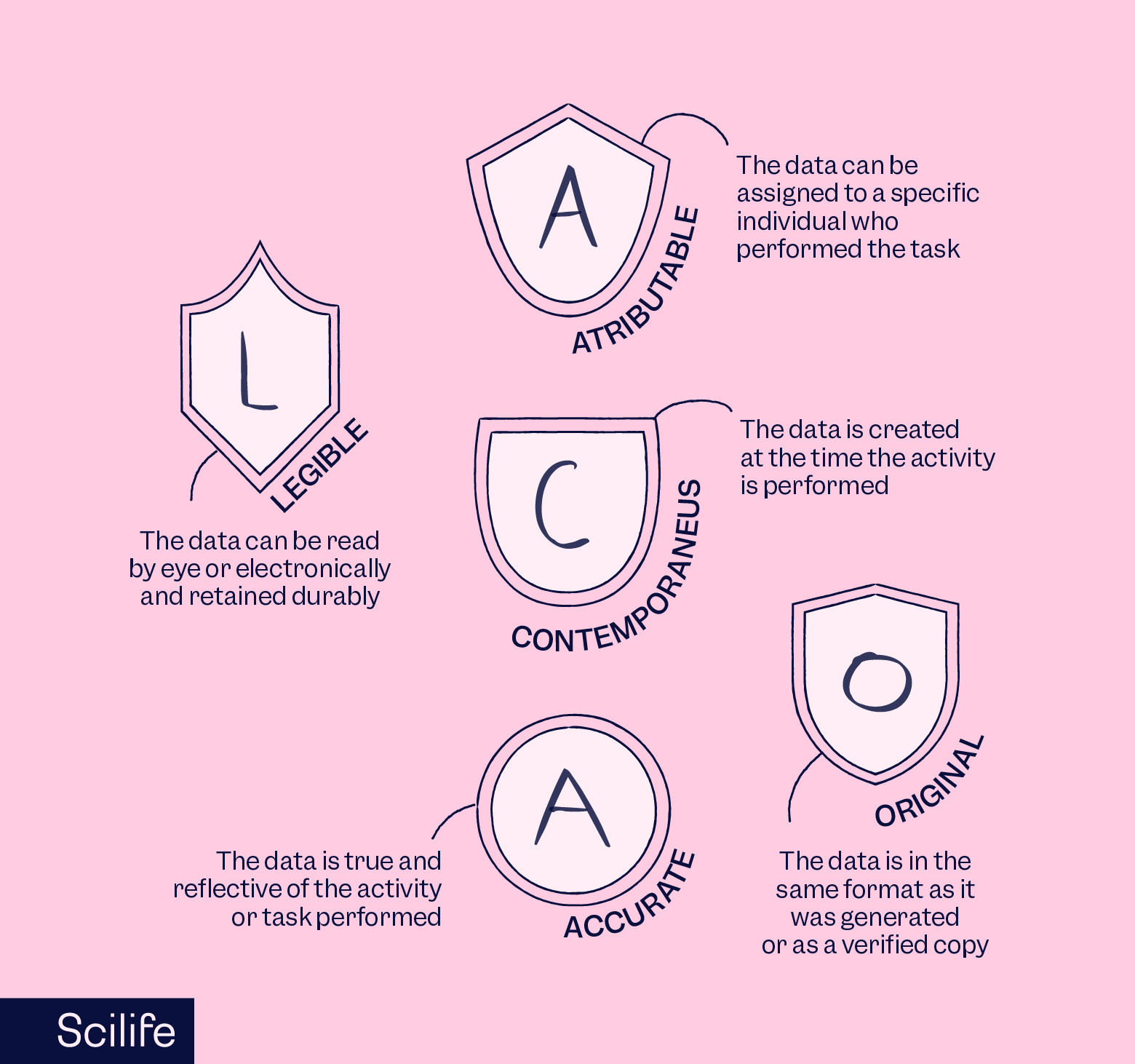
"Data integrity refers to the completeness, consistency, and accuracy of data. Complete, consistent, and accurate data should be attributable, legible, contemporaneously recorded, original or a true copy, and accurate (ALCOA)."
The ALCOA acronym is commonly used in the context of data integrity and stands for:
- Attributable: Data should be traceable to the source, with clear identification of who performed the action or generated the data.
- Legible: Data should be easily readable and not subject to misinterpretation.
- Contemporaneous: Data should be recorded in real-time as the events occur, rather than entered at a later date.
- Original or a true copy: Data should be either the primary record or an authenticated copy of the primary record.
- Accurate: Data should be error-free and reflect the actual information.
The FDA expects regulated companies to adhere to these principles to ensure the reliability and trustworthiness of the data generated in their operations. Failure to maintain data integrity can lead to serious regulatory consequences, including enforcement actions such as warning letters, fines, and other regulatory sanctions.
Why are regulators focusing on data integrity?
As a government agency, U.S. FDA regulators place a significant focus on data integrity due to its pivotal role in ensuring the reliability, accuracy, and trustworthiness of data within regulated industries. The emphasis regulatory agencies on data integrity are driven by the agency’s responsibility to protect the public interest and several other critical factors as follows:
Patient safety
Patient safety is paramount to regulators, hence they focus on data integrity issues to safeguard clinical study data, quality control data, and other QMS records against deliberate data manipulation or misrepresentation to:
- Pass unsafe products as safe,
- Hide potential risks, and
- Hide consistency and reproducibility challenges in the product.
By focusing on data integrity issues, regulators promote global harmonization for international collaboration to enforce quality standards.
Decision making
If there are underlying data integrity issues within an organization, then data-driven decisions will be as inaccurate as the data. Therefore, regulators focus on data integrity to ensure the credibility of data-based go-no-go decisions.
Technological advances
Advances in technology, including electronic data systems, eQMS, analytics, and interconnected databases, have introduced new challenges and opportunities. Regulators focus on adapting standards to address the complexities of modern technological environments, ensuring that data integrity is maintained in the face of evolving tools and methodologies.
What's the cost of poor data integrity?
The cost of poor data integrity in regulated industries can be substantial and can impact various aspects of an organization. The actual cost may be difficult to quantify and may be incurred at a much later time point after the data integrity issue is identified or reported.
For example, a whistleblower alleged that Pfizer falsified data and failed to promptly pursue reports of adverse events in its COVID-19 vaccine trial. At a later time point, the State of Texas filed a petition against Pfizer Inc. in public interest in which the state sought injunctive relief and monetary relief of greater than $1,000,000, including civil penalties, reasonable attorney’s fees, litigation expenses, restitution, and costs. In its petition, the State of Texas mentioned that Pfizer’s clinical studies reported the efficacy of covid-19 vaccine as “95% effective”.
However, more people died due to COVID-19 in 2021 when the Pfizer vaccine was available as compared to the year 2020 when the vaccine was not available. Therefore, it turned out that the natural immune response ensured better patient safety over the Pfizer vaccine. In this case, the actual costs were incurred by Pfizer later as an aftermath of data integrity issues reported by a whistleblower and the pandemic deaths occurred in 2021. The incident also raised questions on the credibility of ex-regulators involved in promoting the Pfizer vaccine and suppressing the Twitter content for undue commercial interests.
In another instance, Indian drug major Ranbaxy is known to have pleaded guilty and agreed to pay a $ 500 million fine to settle criminal and civil charges about data integrity with the US Department of Justice. The consequence of the incident was such that today the company named Ranbaxy no longer exists. Owing to the loss of reputation, hefty fines, and never-ending legal implications, the promoters had to sell off the company to Daiichi Sankyo and later the unit was bought out by Sun Pharma.
Therefore, the consequences of compromised data integrity include anything ranging from regulatory actions such as warning letters, fines, production or marketing injunctions, product recalls, legal consequences, and so on. Additionally, the cost of addressing regulatory issues and implementing corrective measures can be extremely expensive. Apart from the monetary implications data integrity issues also result in a loss of reputation, and supply chain disruption.
How can life sciences ensure data integrity compliance?
The hefty costs associated with data integrity issues can be avoided by implementing robust systems, practices, and a cultural mindset that prioritizes the accuracy, consistency, and reliability of data. Here are key strategies and measures that life sciences organizations can adopt:
Establish a data governance framework
Develop and implement a comprehensive data governance framework that defines roles, responsibilities, and processes for managing data across the organization. This includes policies for data ownership, data stewardship, and clear procedures for data handling.
Implement strong documentation practices
Emphasize meticulous documentation practices for all aspects of data generation, analysis, and reporting. Ensure that procedures are well-documented, data entries are contemporaneously recorded, and there is a clear audit trail for any changes made to data.
Provide adequate training
Train personnel on the importance of data integrity and the specific procedures and practices that ensure compliance. This includes training on relevant regulations, quality management systems, and the organization's specific data integrity policies.
Use electronic systems with audit trails
Implement electronic systems with built-in audit trails that capture and record all changes made to data. These systems provide transparency and traceability, supporting data integrity by preventing unauthorized access and ensuring the accuracy of data records.
Validate computerized systems
Regularly validate computerized systems and software used in data generation and analysis to ensure they meet regulatory requirements and perform as intended. This includes validating analytical instruments, laboratory information management systems (LIMS), and other relevant technologies.
Ensure data security
Implement robust data security measures, including access controls, encryption, and regular cybersecurity assessments. Protecting data from unauthorized access or tampering is essential for maintaining data integrity.
Implement change control procedures
Establish and adhere to change control procedures to manage any changes to systems, processes, or methods that could impact data integrity. Changes should be documented, justified, and assessed for their potential impact on data reliability.
Adopt risk-based approaches
Implement risk-based approaches to data integrity, identifying and mitigating potential risks to data quality. Conduct risk assessments to prioritize areas that require additional scrutiny and implement controls accordingly.
Conduct regular audits and monitoring
Conduct regular internal audits and monitoring activities to assess compliance with data integrity policies and procedures. This includes assessing documentation practices, data security measures, and adherence to standard operating procedures.
Cultivate a culture of compliance
Foster a culture that values data integrity and compliance with regulatory requirements. Encourage open communication, reporting of concerns, and a commitment to continuous improvement in data management practices.
Ensure supplier and vendor oversight
If there are underlying data integrity issues within an organization, then data-driven decisions will be as inaccurate as the data. Therefore, regulators focus on data integrity to ensure the credibility of data-based go-no-go decisions.
Stay informed about regulatory guidance
Stay informed about evolving regulatory guidance on data integrity. Regularly review and update policies and procedures to align with the latest industry standards and regulatory expectations.
By adopting these strategies, life sciences organizations can create a strong foundation for data integrity compliance, minimizing the risk of regulatory issues, ensuring the reliability of data, and contributing to the overall quality and safety of products and processes.
Conclusion
In conclusion, the heightened focus on data integrity by regulators, exemplified by the U.S. Food and Drug Administration (FDA), stems from its pivotal role in ensuring the reliability, accuracy, and trustworthiness of data within regulated industries. This emphasis is underscored by the FDA's "Data Integrity and Compliance with CGMP" guidance, outlining key principles encapsulated in the ALCOA acronym.
Regulators prioritize data integrity to safeguard patient safety, prevent the misrepresentation of clinical studies and quality control data, and promote global harmonization for enforcing quality standards. The cost of poor data integrity, as illustrated by high-profile cases like Pfizer and Ranbaxy, encompasses regulatory actions, legal consequences, reputational damage, and supply chain disruptions.
To mitigate these risks, life sciences organizations must implement robust systems, practices, and a culture that prioritizes data accuracy. Key strategies include establishing a comprehensive data governance framework emphasizing strong documentation practices; providing adequate training; using electronic systems with audit trails; validating computerized systems; ensuring data security, implementing change control procedures; adopting risk-based approaches; conducting regular audits; cultivating a culture of compliance; overseeing suppliers and vendors, and staying informed about evolving regulatory guidance. These measures collectively contribute to data integrity compliance, reducing the likelihood of regulatory issues and enhancing overall product and process quality and safety.