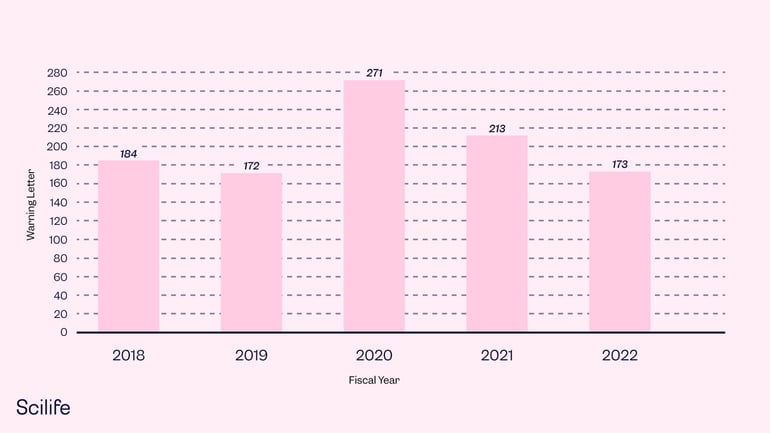
Warning Letter
The FDA allows firms to take voluntary and prompt corrective actions before initiating an enforcement action. These practices are termed Advisory Actions, and their corresponding letters are called Advisory Action Letters. The types of letters can differ depending on the nature of the violation and be categorized as Untitled Letters and Warning Letters.
Untitled Letters mention violations that do not meet the regulatory significance threshold for Warning Letters. However, they serve as an initial notification to companies that the FDA is aware of their violations.
Warning Letters are sent to firms to request voluntary compliance before an FDA notice. These letters are issued when the company's violations of regulatory significance may lead to the FDA’s enforcement action if not promptly and adequately corrected. Any warning letter requires prompt voluntary compliance with the Act.
Importantly, the FDA is not required to send a warning letter before initiating an enforcement action. Warning letters are also not a final action by the agency. These letters communicate the FDA’s position but do not guarantee that the agency will take enforcement action.
Warning letters are used to notify a manufacturer when the manufacturer violates FDA regulations. In most cases, notifications appear in the form of a warning letter.
There are four different types of FDA warning letters:
General FDA Warning Letters
This letter identifies the violation, which can be for example a lack of Good Manufacturing Practices, problems with claims about features of the product, or insufficient or missing directions for use. Additionally, the FDA requires the addressed manufacturer to fix and eliminate the problem(s). When a company receives a warning letter, they also need to respond to the agency within fifteen (15) business days – not calendar days – with an action plan to correct the violations.
Firms are also allowed to dispute whether or not they violate the Act and can provide supporting documentation for reconsideration. The FDA will review any supporting evidence and the firm’s action plan to determine whether these actions are sufficient to correct the identified violations. Additionally, the FDA will monitor the firm’s future activities to ensure that they stay in compliance with all applicable regulations. FDA warning letters might be subject to subsequent interaction between the FDA and the letter recipient (i.e., the manufacturer) that might change the regulatory status of the identified issues.
All warning letters are posted on the FDA’s website for public access. In addition, individuals can go to the FDA warning letters website to filter and review warning letters submitted as recently as today.
Tobacco Retail Warning Letters
The Family Smoking Prevention and Tobacco Control Act, also known as the “Tobacco Control Act,” periodically conducts compliance inspections of tobacco retailers. These compliance checks are conducted to review a retailer’s compliance with the Tobacco Control Act and its regulations regarding all tobacco products, including cigarettes and smokeless tobacco.
If a warning letter is issued to a tobacco retailer for violations observed during a compliance inspection, they can be found in the Compliance Check Inspections database. The warning letters that are issued by the CTP can be found on the FDA’s Warning Letter page. The Tobacco Retailer Warning Letters overview also briefly explains what retailers need to do if they have received such a warning letter.
Drug Marketing and Advertising Warning Letters
These letters are issued by the FDA’s Division of Drug Marketing and Communications and Drug Warning Letters. Some of these letters need to be redacted to remove or hide confidential information. Sometimes, letters can be sent electronically, for example, to websites called “Cyber” that sell online prescription drugs, which might be illegal. Basically, this type of warning letter warns website operators that they may be engaging in illegal activities; and the FDA informs them of the laws that govern prescription drug sales. All other Warning Letters issued by CTP can be found on the FDA’s Drug Marketing and Advertising Warning Letters page.
Warning Letter Close-Out Letter Program
This program only applies to letters that were issued on or after the 1st of September 2009.
The FDA might issue a Warning Letter Close-Out Letter Program (often just called a “close-out letter”) once the agency completes the evaluation of taken actions by the company in response to a Warning Letter.
The close-out letter should not be issued based on representations, and all corrective actions should have been completed and verified by the FDA. Generally, the FDA conducts a follow-up inspection as a standard practice to verify corrections that have been implemented. If the warning letter contains violations that cannot be corrected by their nature, then the FDA will not issue a close-out letter.
Additional resources

How to Implement the Continuous Improvement Cycle | Scilife
Even an organization with stellar leadership and a solid core of employees experiences hiccups from time to time. Despite having assembled all the ...

How to assess and enhance your Quality Management Maturity | Scilife
As the life sciences industry becomes increasingly regulated and competitive, quality management has become more vital than ever. Are you confident ...

Best Quality Management Software (QMS) for Life Sciences | Scilife
The right electronic Quality Management System (eQMS) can help strengthen your compliance processes and build a culture of quality within your ...

How to write a good quality plan for medical devices | Scilife
In life sciences, especially if you’re in the medical device industry it becomes harder to manage projects in accordance with your company’s quality ...
Turn quality into your brightest asset with Scilife
