EMEA Office
Louizalaan 489
1050 Brussels
Belgium

Overwhelmed by the need to adapt your operating models to meet the dynamic demands of patients, stakeholders and regulatory authorities?
Don’t limit your growth opportunities and empower your innovation.
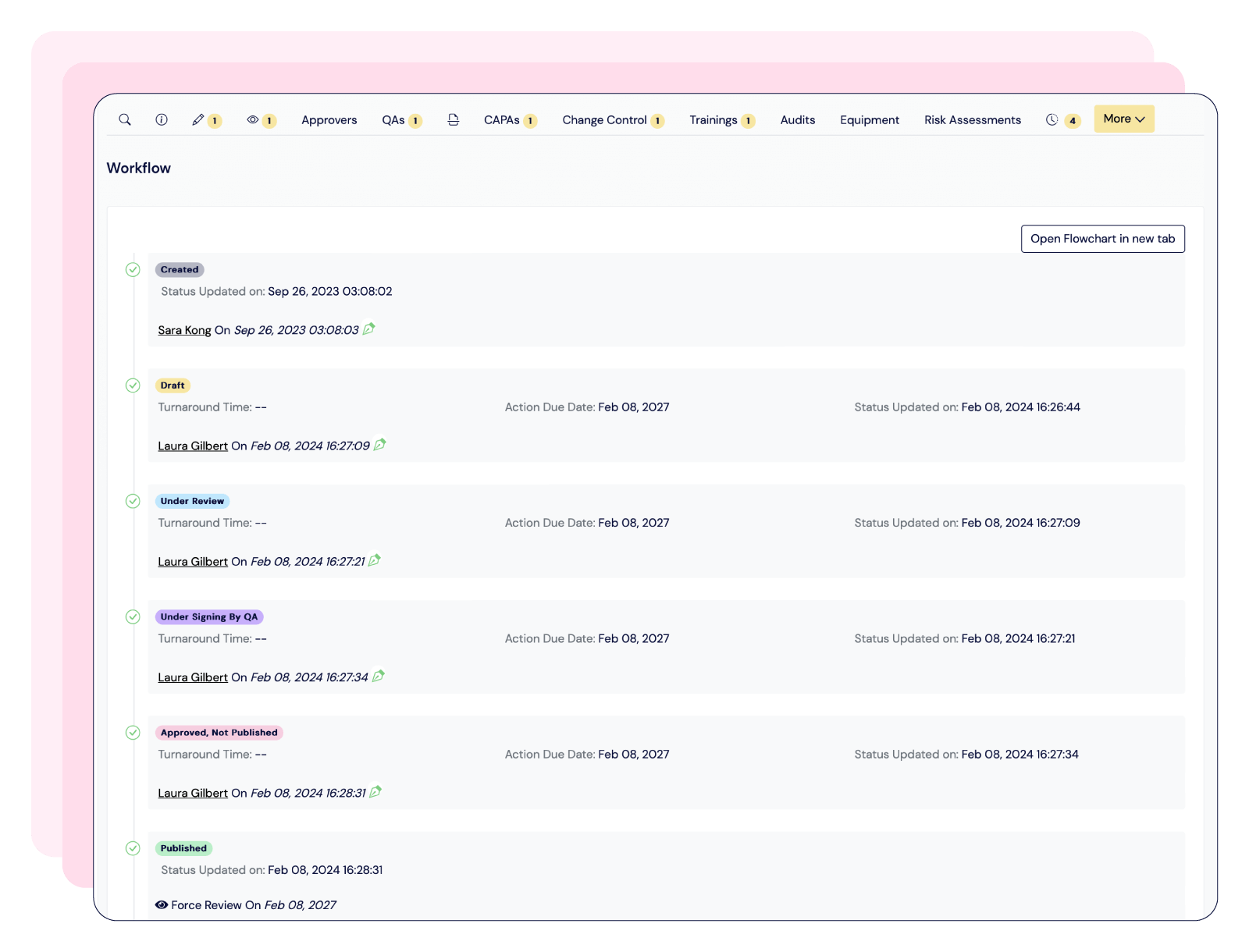
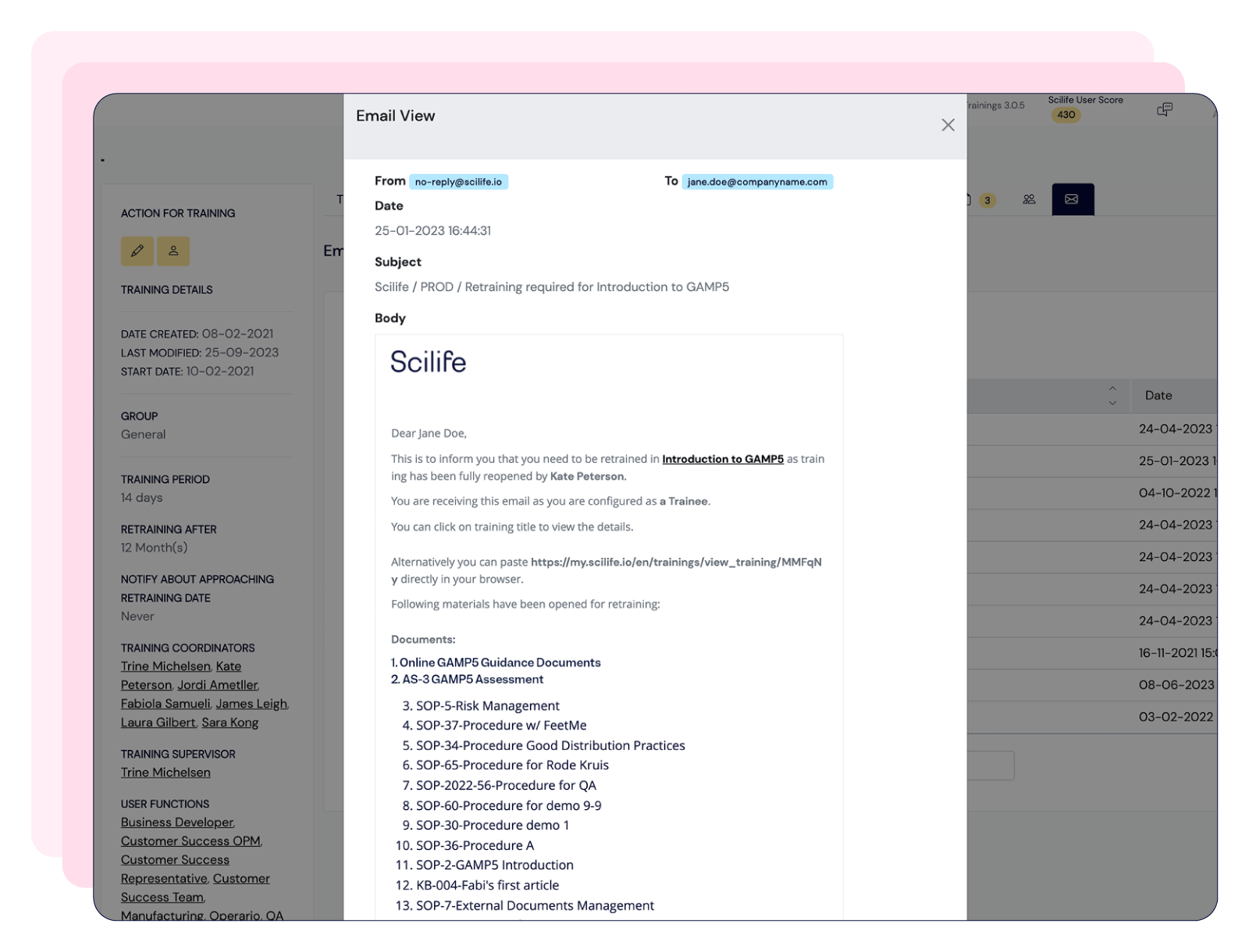
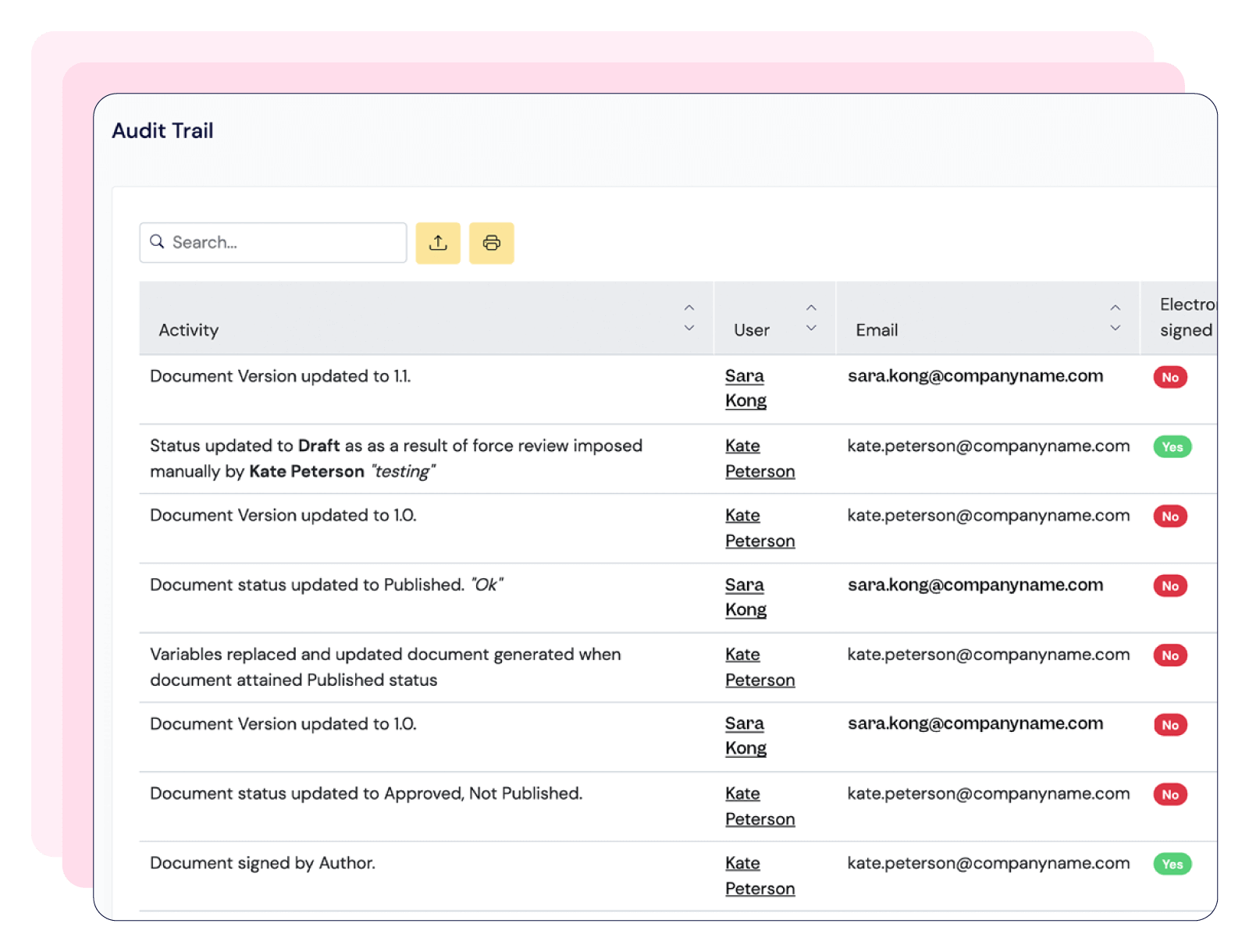
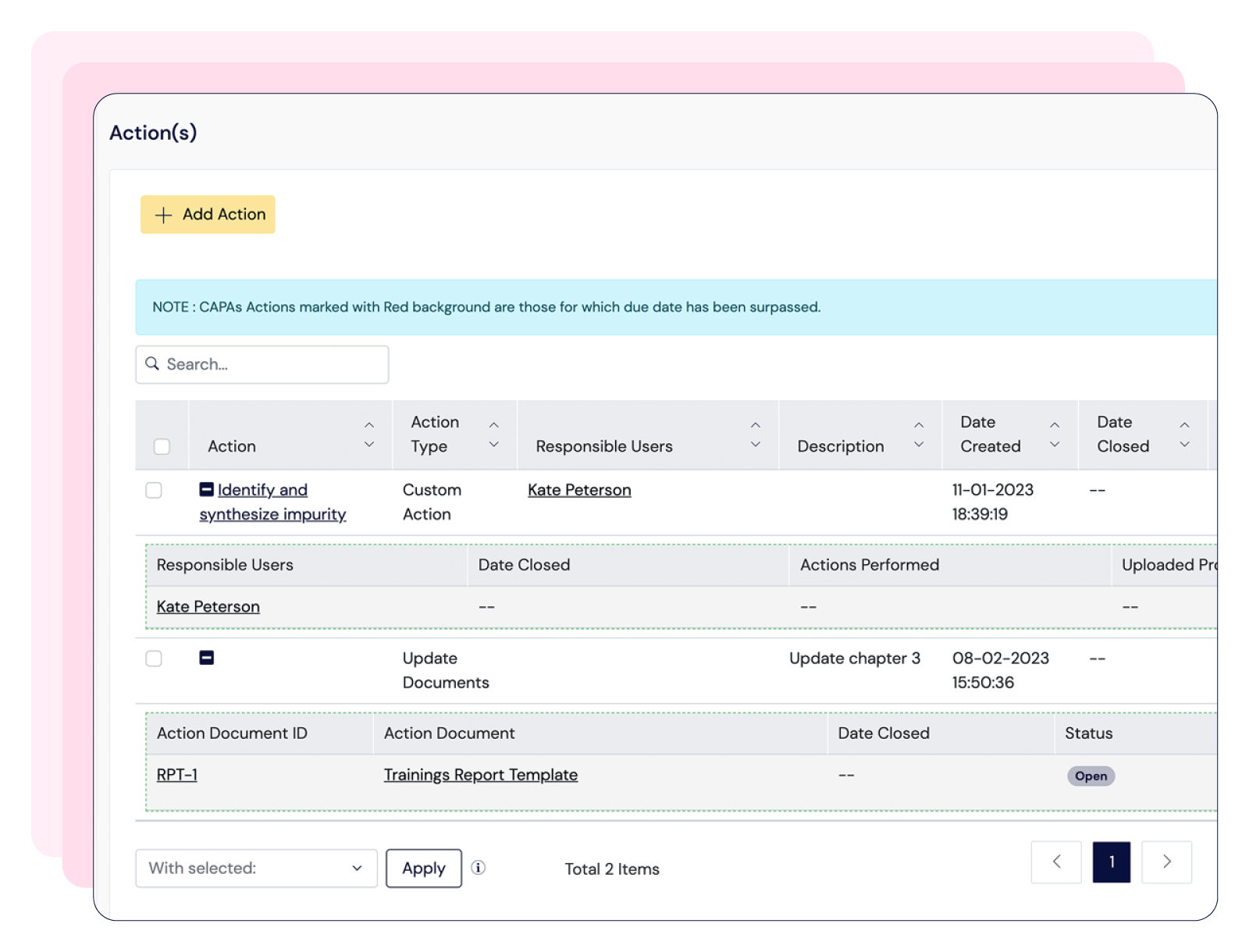
Scilife provides an all-in-one QMS that ensures a centralized and efficient document management system with 21 CFR Part 11-compliant electronic signatures; training management system with role-based trainings and automated notifications; and an audit management solution linked to CAPAs, Quality Events and Risk Management.
“Scilife has made our life easier in so many ways. One of the things I like the most is how it integrates different processes. It gives a bigger picture of everything and allows us to keep track of deviations and see opportunities for improvement.”
 Daniele Scalco,
Daniele Scalco,
Quality Assurance and Regulatory Affairs Manager at Idevax
EMEA Office
Louizalaan 489
1050 Brussels
Belgium
US Office
Scilife Inc.
228 E 45th St. RM 9E
New York, NY 10017
EMEA Office
Louizalaan 489
1050 Brussels
Belgium
US Office
Scilife Inc.
228 E 45th St. RM 9E
New York, NY 10017
Copyright 2024 Scilife N.V. All rights reserved.