"Scilife offers all the required functionalities in a flexible, cloud-based package which complies with the GxP guidelines."







Implement a smart quality software solution and follow all the regulatory requirements—controlling your documents' lifecycle, conducting internal audits, and tracking employees' expertise.
Clinical research is often perceived as costly and time-consuming. Invest in a smart QMS solution and improve data quality, patient safety, and access to clinical trial processes.

Manage workload, timelines, and resources wisely using a smart quality tool that helps you improve performance.
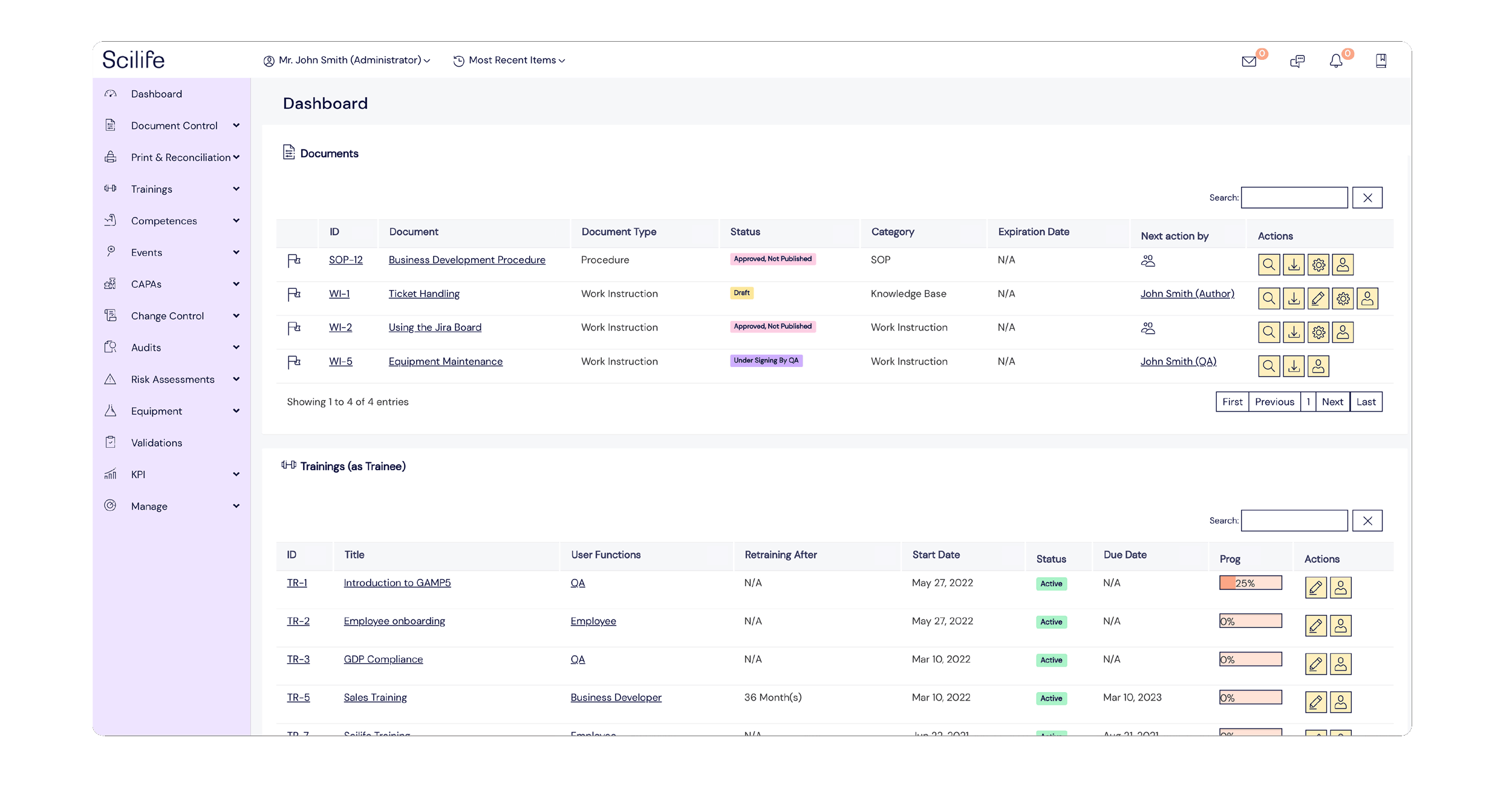
Popular Features
How we support other companies like yours


"Scilife offers all the required functionalities in a flexible, cloud-based package which complies with the GxP guidelines."


"We have been using Scilife to monitor the quality metric KPI's closely. It has enabled us to narrow down the root causes for several deviations with an ultimate benefit of improving quality and reducing costs."


"The ISO9001:2015 certification is a key demonstration of a fully integrated quality approach which reinforces the efficiency and robustness of our development programs. We achieved such a key objective with the help of Scilife."
Customers rated Scilife the Best Pharma and Biotech QMS Software again in the G2 Summer 2022 report.
This ranking system considers both user satisfaction and market presence of the software, compared to similar products, placing Scilife as the leader in the Pharma & Biotech category.


EMEA Office
Louizalaan 489
1050 Brussels
Belgium
US Office
Scilife Inc.
228 E 45th St. RM 9E
New York, NY 10017
EMEA Office
Louizalaan 489
1050 Brussels
Belgium
US Office
Scilife Inc.
228 E 45th St. RM 9E
New York, NY 10017
Copyright 2025 Scilife N.V. All rights reserved.