"You can have an event that triggers a CAPA, that CAPA can trigger an update of your document, that document update triggers a new training, and so on. So that's a huge benefit of the system."



Medicinal Cannabis regulation is still under discussion and can be tricky to manage! Stay ahead of your competitors by centralizing all your documents and processes in a single smart quality platform.

A well-established quality culture, with a good production and control strategy, guarantees a robust value chain that will allow you to meet all the relevant specifications and regulatory requirements.

Make sure you have a strong, efficient quality system by digitalizing all your processes and documents. That way you can invest time in risk prevention and mitigation instead.
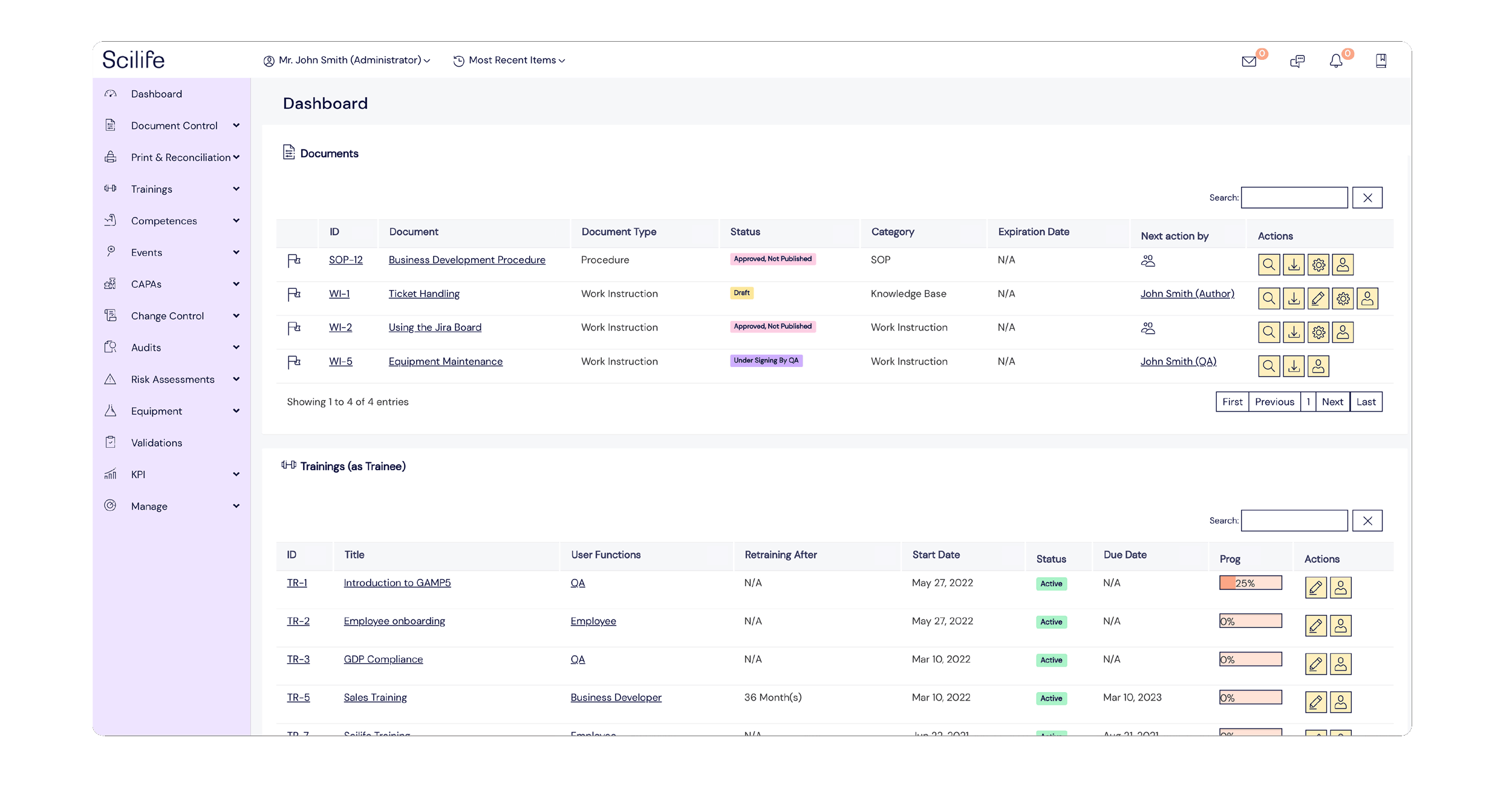
Popular Features
Here’s where Scilife can help you



‘Doing an audit with Scilife at your fingertips was amazing! Being able to pull up documents at a moment's notice and show them on the screen, showing them batch records and training records and how they are all linked. I think the auditors were just blown away at how easily we could navigate everything. They were just so impressed that it was so transparent!’


"Scilife really offers everything we want in an eQMS. If there’s something we can’t do with the platform yet we’ll either find an effective workaround, or, as is usually the case, Scilife will tell us that it’s a new feature already in the pipeline. Scilife is remarkably eager to improve by listening to their clients."


"We have been using Scilife to monitor the quality metric KPI's closely. It has enabled us to narrow down the root causes for several deviations with an ultimate benefit of improving quality and reducing costs."


"Scilife’s support is second to none in the market. Not only does the Scilife team react promptly to tickets, but the Scilife support team listens to my ideas and dedicates time to fixing every issue, improving the system as a whole."



‘Doing an audit with Scilife at your fingertips was amazing! Being able to pull up documents at a moment's notice and show them on the screen, showing them batch records and training records and how they are all linked. I think the auditors were just blown away at how easily we could navigate everything. They were just so impressed that it was so transparent!’


"Scilife really offers everything we want in an eQMS. If there’s something we can’t do with the platform yet we’ll either find an effective workaround, or, as is usually the case, Scilife will tell us that it’s a new feature already in the pipeline. Scilife is remarkably eager to improve by listening to their clients."


"We have been using Scilife to monitor the quality metric KPI's closely. It has enabled us to narrow down the root causes for several deviations with an ultimate benefit of improving quality and reducing costs."


"Scilife’s support is second to none in the market. Not only does the Scilife team react promptly to tickets, but the Scilife support team listens to my ideas and dedicates time to fixing every issue, improving the system as a whole."
Customers rated Scilife the Best Pharma and Biotech QMS Software again in the G2 Summer 2022 report.
This ranking system considers both user satisfaction and market presence of the software, compared to similar products, placing Scilife as the leader in the Pharma & Biotech category.


EMEA Office
Louizalaan 489
1050 Brussels
Belgium
US Office
Scilife Inc.
228 E 45th St. RM 9E
New York, NY 10017
EMEA Office
Louizalaan 489
1050 Brussels
Belgium
US Office
Scilife Inc.
228 E 45th St. RM 9E
New York, NY 10017
Copyright 2025 Scilife N.V. All rights reserved.