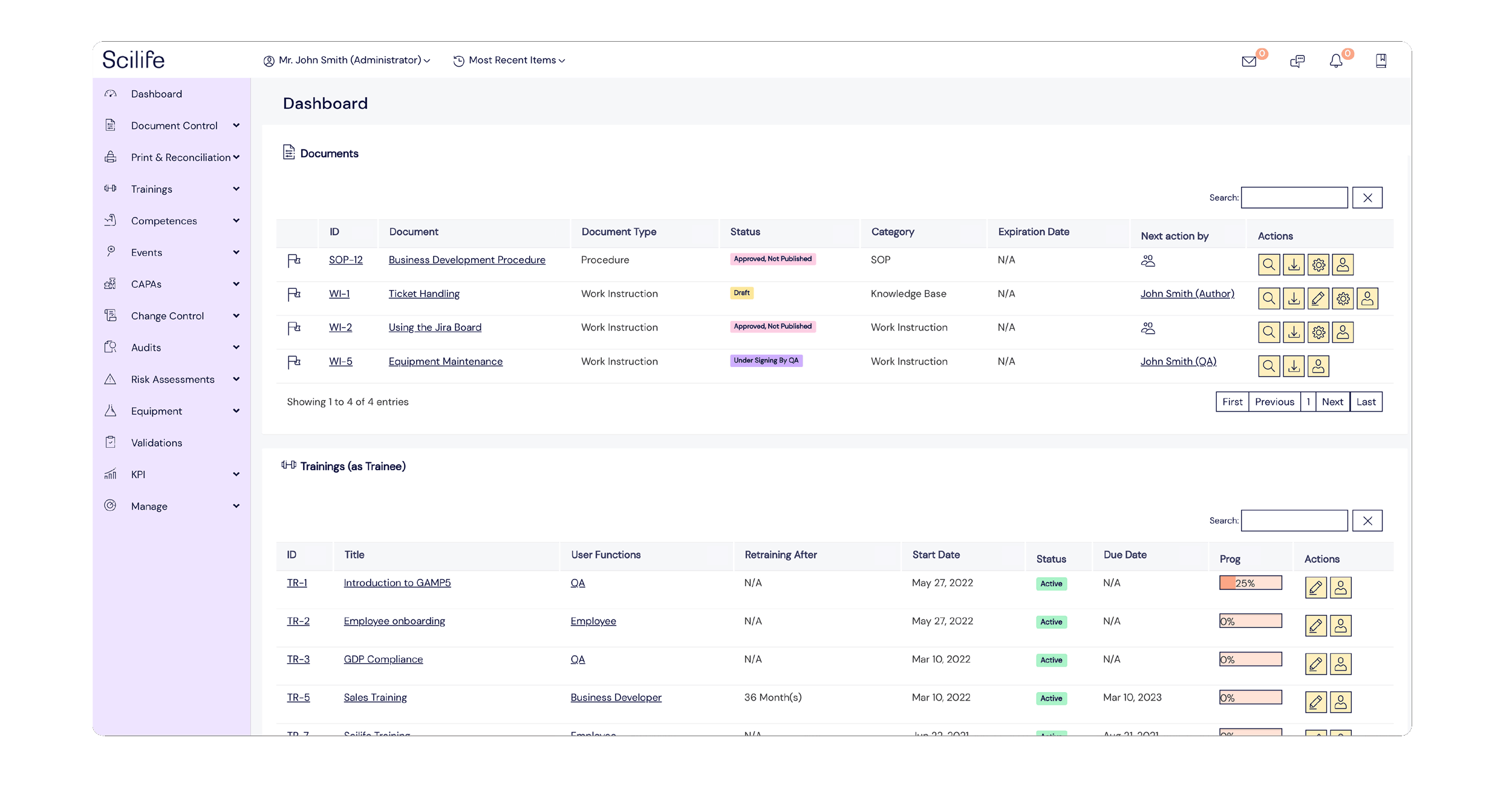
‘A paper-based QMS is a major pain point for anyone. Having to do manual signoffs, maintaining paperwork, tracking missing updates, documents or trainings is a whole lot more time consuming. We used to pass paper along different people's desks and have to wait. With paper in general, there are associated risks.’

.png?width=252&height=50&name=05_Amnovis-Logo-Mono-Zwart@4x%20(1).png)