EMEA Office
Louizalaan 489
1050 Brussels
Belgium
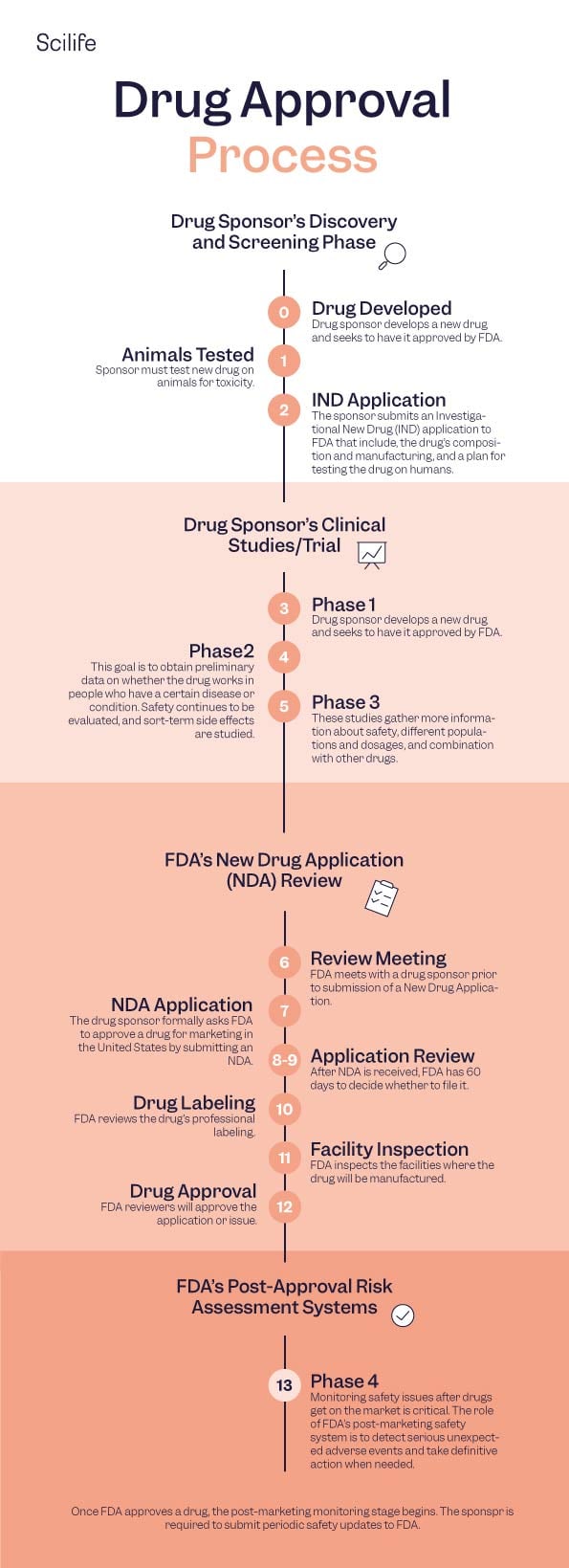
01. A Discovery
01. B Development
02. A In vitro
02. B In vivo
03. A Clinical Trial Design
03. B Clinical Trial Phases
03. C Investigational New Drug (IND) process
03. D FDA IND Review Team
03. E Drug Approval
04. A New drug application
04. B FDA Review
04. C FDA Approval
04. D FDA Advisory Committees
05. A Supplemental Application
05. B INDs for marketed drugs
05. C Manufacturer inspections
05. D Drug advertising
05. E Generic drugs
05. F Reporting problems
05. G Active surveillance
05. H Adverse event reporting
Complete your download by reading:
EMEA Office
Louizalaan 489
1050 Brussels
Belgium
US Office
Scilife Inc.
228 E 45th St. RM 9E
New York, NY 10017
EMEA Office
Louizalaan 489
1050 Brussels
Belgium
US Office
Scilife Inc.
228 E 45th St. RM 9E
New York, NY 10017
Copyright 2025 Scilife N.V. All rights reserved.