Looking to get CE approval for medical devices? Navigating the regulatory landscape is essential for medical device manufacturers aiming to market their products in Europe. As one of the most important regulatory markets in the world, the EU stands at the forefront of regulatory development and innovation. The CE certification mark is a critical component of this process.
This guide offers a comprehensive overview of the process for obtaining CE approval for medical devices, its importance, the countries that require or recognize it, the types of devices that need it, the steps involved, and answers to frequently asked questions. By understanding the CE certification mark and the associated regulations, manufacturers can ensure their medical devices meet European standards and are ready for market entry.
What's the CE Certification Mark?
The CE certification mark, often referred to as the CE mark, is a symbol that indicates a product complies with the essential health, safety, and environmental protection requirements established by European legislation. "CE" stands for "Conformité Européenne," which translates to "European Conformity." When a product bears the CE mark, it meets the applicable directives and regulations enforced by the European Union (EU).
The CE mark is not a quality indicator or a certification of origin; rather, it demonstrates that the product can be legally sold within the European Economic Area (EEA), including the EU member states and EFTA countries (Iceland, Norway, Liechtenstein).
Why is CE approval for medical devices important?
CE approval for medical devices is crucial for several reasons:
Legal Market Access
The primary importance of the CE mark is that it provides legal access to the European market. Medical devices cannot be legally sold in the EEA without the CE mark.
Compliance with Regulations
Obtaining the CE mark ensures that a medical device complies with all relevant EU regulations, specifically the Medical Device Regulation (MDR) (EU 2017/745) and the In Vitro Diagnostic Regulation (IVDR) (EU 2017/746). This compliance is essential for safeguarding public health and ensuring that products are safe and effective for their intended use.
Competitive Advantage
Possessing a CE mark can offer a competitive advantage, as it demonstrates to healthcare providers and patients that the product has met rigorous European standards. This can enhance the credibility and marketability of the device, not just in Europe but in other markets around the world that leverage the CE mark.
Global Recognition
While the CE mark is specific to the EEA, it is recognized globally and often seen as a product quality and safety benchmark. This recognition can facilitate market entry in other regions, as some countries accept or use the CE marking system as a model for their regulatory frameworks.
What Countries Require or Accept CE Marking?
The CE marking is mandatory for products sold in the European Economic Area, which comprises the 27 EU member states and the three EFTA countries: Iceland, Norway, and Liechtenstein. Additionally, Switzerland and Turkey mutually agree with the EU to accept CE-marked products in their markets.
Beyond these countries, many other regions recognize the CE mark directly or as part of their regulatory processes. For instance, some Middle Eastern, Asian, and Latin American countries may accept CE-marked products, making it easier for manufacturers to enter these markets.
Which Medical Devices Require CE Marking?

All medical devices intended for sale within the EEA must bear the CE mark. This includes many products, from simple bandages to complex imaging systems and diagnostic equipment. The Medical Device Regulation (MDR) classifies medical devices into four categories based on the level of risk:
- Class I: Low-risk devices (e.g., bandages, stethoscopes).
- Class IIa: Medium-risk devices (e.g., dental fillings, hearing aids).
- Class IIb: Higher-risk devices (e.g., surgical lasers, infusion pumps).
- Class III: High-risk devices (e.g., pacemakers, heart valves).
Each classification has specific requirements for conformity assessment, which dictates the pathway to obtaining the CE mark – but all device classes require the CE mark to be sold in the EEA.
How To Obtain CE Approval for Medical Devices
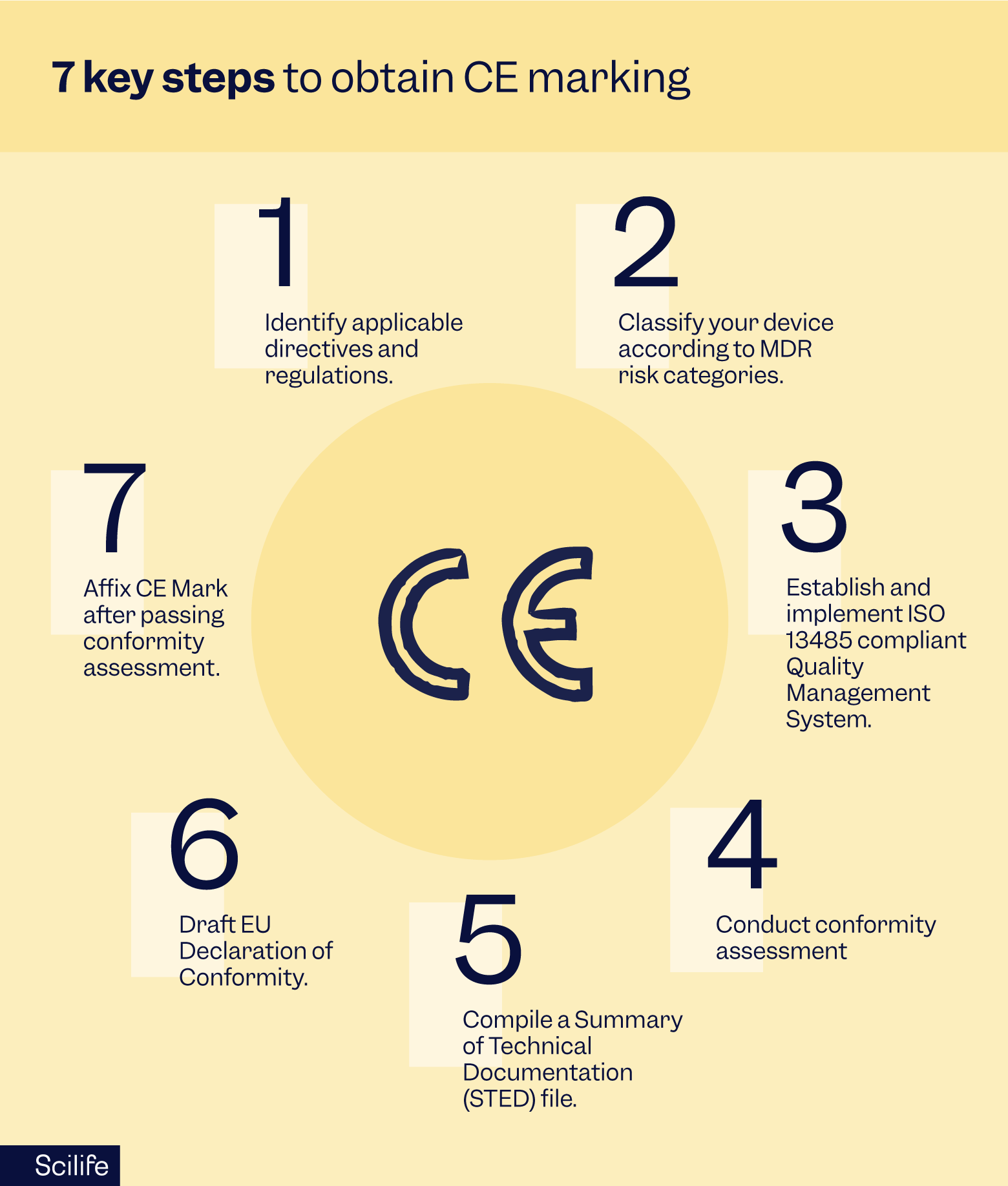
The medical device industry works with seven steps to obtain CE marking:
- Identify applicable directives and regulations and determine which directives apply to your medical device (e.g., MDR, IVDR).
- Classify your device according to the risk categories outlined in the MDR.
- Establish a Quality Management System (QMS) and implement a QMS that complies with ISO 13485. While ISO 13485 is not mandatory for the implementation of a quality management system, it is by far the most complete and simplest way to a compliant QMS.
- Conduct your conformity assessment: a notified body must assess the conformity for higher-risk devices (Class IIa, IIb, and III). Self-certification may be sufficient for lower-risk devices (Class I).
- Compile your STED (Summary of Technical Documentation) file: Prepare comprehensive documentation that includes a detailed description of the device, design and manufacturing processes, risk management, clinical data, and compliance with applicable standards.
- Draft an EU Declaration of Conformity which states that your medical device complies with the relevant EU regulations.
- Affix your CE Mark: Once the device has passed the conformity assessment, the CE mark can be affixed to the product, packaging, and accompanying documentation.
The process can take time, but breaking it down into steps makes it much easier. Following these key steps will simplify getting CE approval for medical devices.
Critical Regulations for CE mark
- Medical Device Regulation (MDR) (EU 2017/745): governs the manufacturing and marketing of medical devices in the EU.
- In Vitro Diagnostic Regulation (IVDR) (EU 2017/746): applies to in vitro diagnostic medical devices. Some in vitro diagnostic devices still work under the In Vitro Diagnostic Directive (IVDD; Directive 98/79/EC of the European Parliament and the Council of 27 October 1998 on in vitro diagnostic medical devices) but will be required to transition to the IVDR within a few years.
- ISO 13485: An international standard that specifies requirements for a QMS specific to the medical devices industry.
FAQs About CE Certification Mark
What is the difference between the CE mark and FDA approval?
The CE mark and FDA approval are both regulatory processes but serve different markets. The CE mark allows a medical device to be sold in the EEA, while FDA approval is required for sale in the United States. Each has its own set of standards and procedures. While the two are similar in many ways, one cannot substitute another.
How long does it take to obtain the CE mark?
The timeline for obtaining the CE mark can vary widely depending on the device's classification and the complexity of the conformity assessment process. For devices requiring notified body intervention, the process can take several months to a few years.
Can a non-EU manufacturer apply for the CE mark?
Yes, a non-EU manufacturer can apply for the CE mark. However, they must appoint an Authorized Representative based in the EU to act on their behalf in the regulatory process.
What happens if my medical device does not comply with CE marking requirements?
Non-compliance with CE marking requirements can result in penalties, including fines and product recalls. The device can only be legally sold in the EEA once it meets all necessary regulations.
Is the CE mark valid indefinitely?
No, the CE mark is not indefinitely valid. Its validity depends on the type of device and the conformity assessment procedure used. Regular surveillance audits and re-certification may be required to maintain it.
Do I need to notify the authorities if there are changes to my device?
Yes, significant changes to the device design, intended use, or manufacturing process may require re-assessment and notification to the relevant authorities or notified body.
Where should the CE mark be placed?
The CE mark must be visibly, legibly, and indelibly affixed to the medical device, its packaging, and any accompanying documents, such as the instructions for use.
Learn how Scilife Smart QMS for medical devices takes the stress out of getting your CE mark and smoothly launches your product in the EU market.