Before a drug is suitable for patients, it must pass rigorous testing and cost-benefit analyses.
In this post, we will discuss the journey a new medicinal product must take from the lab to final authorization in the EU.
Continue reading this post to deepen your understanding of the drug development timeline.
What does the drug development process look like?
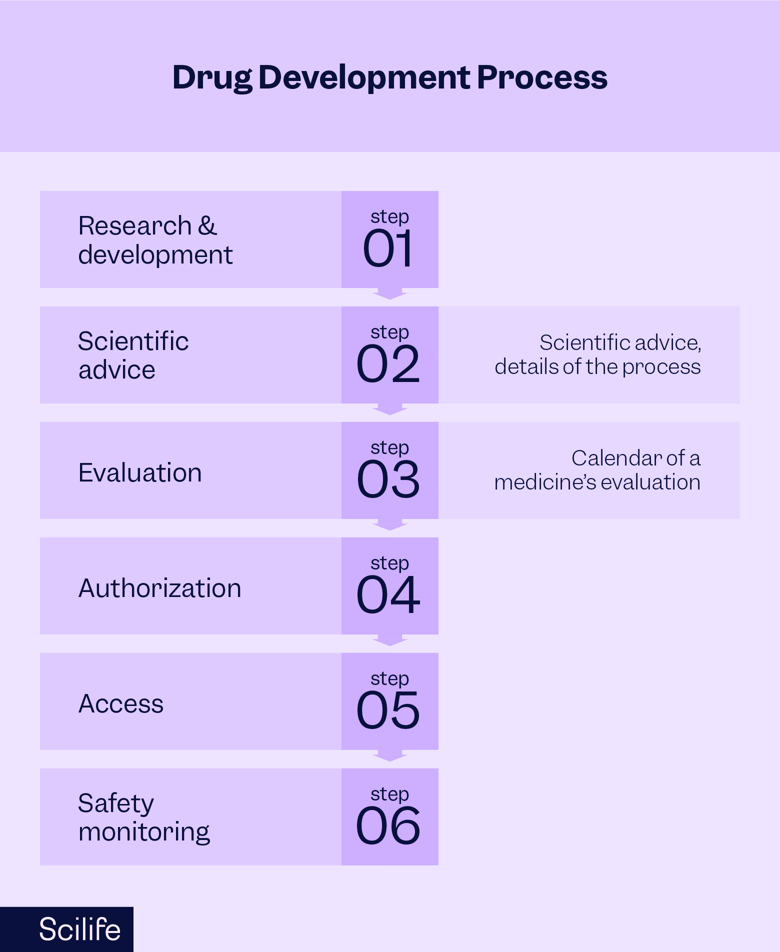
According to the European Medicines Agency (EMA), there are six steps a medicinal product must take.
These include:
- Research and development
- Scientific advice
- Evaluation
- Authorization
- Access
- Safety monitoring
In this process, the EMA’s Committee for Medicinal Products for Human Use (CHMP) will assess each application and make a recommendation to the European Commission (EC).
The EC will then issue a final, legally-binding decision on whether the drug is approved for marketing in the EU.
What are the phases of drug development?
Above we listed the six phases of drug development.
Now let’s go over each one in detail.
1_ Research and development
When it comes to researching new medicinal products, the process typically begins with private biotechnological and pharmaceutical companies. Public institutions, doctors, and academics may be involved as well.
Not all research makes it to development, however. Every year, 10,000s of molecules are investigated—but only a fraction show promise.
And while the EMA supports innovation in the pharmaceutical sector, it cannot force companies to conduct research on specific medicines for specific conditions.
Instead, the EMA highlights areas with demand for new treatments. The goal is to motivate researchers to invest in these fields.
And so begins the research and development phase of drug development. The development process starts by searching for a target on which the compound can act as a drug in the body.
From there, developers can look for molecules that may act as a new active drug substance. Only a few of these molecules will make it to the market.
This is where clinical trials come in. It’s important to note that the process of testing potential new drugs starts in the lab and continues in clinical trials with human volunteers.
To initiate a clinical trial, drug developers must submit applications to competent authorities for authorization. They must also follow international standards and Good Clinical Practices to support the medicine’s marketing authorization.
2_ Scientific advice
Speaking of good practices, drug developers should request scientific advice from the EMA during the development process.
This conversation should touch on the best methods of clinical trial design. The goal here is to generate robust information that shows the new medicine is safe and effective before it is authorized.
Specifically, scientific advice should focus on:
- Quality aspects of the medicine, manufacturing, and testing
- Toxicology and pharmacological tests
- Clinical trial aspects
- Data and statistics
Then, once the clinical trial is completed, developers can submit their application with all the data they’ve collected.
The EMA’s CHMP is responsible for assessing all marketing authorization applications in the EU.
3_ Evaluation
This multi-step phase of the drug development timeline is divided into two sub-processes:
a. Application preparation
Drug developers need to check all the relevant guidance and legislation to comply with the regulatory requirements of the marketing authorization application.
The following data must be collected before submitting the marketing authorization:
- Rationale for the new medicine to the target population
- Mechanism of action
- Quality data
- Pharmacological and toxicological data
- Evidence of compliance with Good Clinical Practices, Good Laboratory Practices, and Good Manufacturing Practices
- Risk management plan
- Summary of product characteristics (SmPC)
- Labeling and packaging
- Plans for follow-up studies
b. Assessment
When following the centralized evaluation process for a new medicine in the EU, developers need to adhere to an active period of up to 210 days.
If a product is authorized using the centralized procedure, it is assessed on an EU-wide basis and approved by the EC.
Conversely, the decentralized procedure allows manufacturers to submit applications to individual EU member states.
In the decentralized procedure, a committee of EMA experts will review the applicant’s evidence and closely scrutinize their application. This committee may recommend inspecting the manufacturing site, the clinical study site, and the pharmacovigilance processes cited in the application.
The purpose of the assessment is to confirm the benefits of the new medicine outweigh the risks at the patient level (in line with the Risk Management Plan).
Key fact: The assessment process is occasionally interrupted by what’s known as a clock-stop. This is a designated time period in which the evaluation process pauses so that the applicant can respond to the regulatory authority’s questions.
Typically, in Europe, there are one or two clock-stops in the drug evaluation process. The first may take place around 120 days. At this point, the applicant has three to six months to answer the CHMP’s questions.
Then, the clock restarts, and the second clock-stop takes place around 180 days of active evaluation. At this time, the applicant has one to three months to address any outstanding issues.
On day 210 of the active evaluation period, the committee may ask the applicant to address any major objections to outstanding issues. Committee members will then share their final opinion on the application.
Applicants who don’t agree with the committee’s final decision may request a reexamination within 15 days of receiving the CHMP’s report.
4_ Authorization
This step of the drug development timeline is fairly straightforward.
With the EMA’s recommendation, the EC is responsible for the marketing authorization of a medicinal product.
Once a new medicinal product is authorized by the EC, it is published in the Union Register.
5_ Access
Now let’s discuss who makes decisions on patient access to medicinal products.
Once a drug receives an EU-wide marketing authorization, decisions about pricing and reimbursement take place at the national and regional level.
Because these choices must be made in accordance with each country’s national health system, the EMA is not involved in decisions surrounding pricing or reimbursement.
The EMA does, however, work with national bodies to help manage these processes.
5_ Safety monitoring
Just because a drug is on the market doesn’t mean safety monitoring can fall to the wayside.
Once a medicinal product is authorized for use in the EU, the EMA and EU member states must monitor its safety constantly. They need to take swift action if new information shows the medicine isn’t as safe as it initially seemed.
The following processes are integral to the safety monitoring phase of the drug development timeline:
Assessing the way risks associated with the drug will be managed and monitored post-authorization
-
Continuously monitoring potential side effects reported by patients and healthcare providers in new clinical studies or scientific publications
-
Regularly reviewing reports submitted by the company that holds the drug’s marketing authorization
-
Evaluating the design and results of any post-authorization safety studies that were required at the time of authorization
Please note that the EMA can also review a medicine (or class of medicines) upon request of an EU member state or the EC. This is part of what’s known as an EU referral procedure.
Conclusion
In conclusion, these are the six key phases of the drug development timeline. We believe that with the above insights in your toolkit, your organization will be ready to experience the full journey a new drug must take. Consider this post your guide for bringing a medicinal product from the lab to final authorization and beyond.