Implementing a Quality Management System is key to ensuring consistency in all your processes and, ultimately, guaranteeing your product quality and safety.
A well-established QMS will allow you to meet both customer and organizational requirements, easing the task of becoming fully compliant and generating confidence with the customers, empowering improvement and growth. Also, it’ll help you prevent any possible mistakes and, consequently, lower costs and waste, identify training opportunities, and set a common direction for the whole organization.
How to implement a Quality Management System?
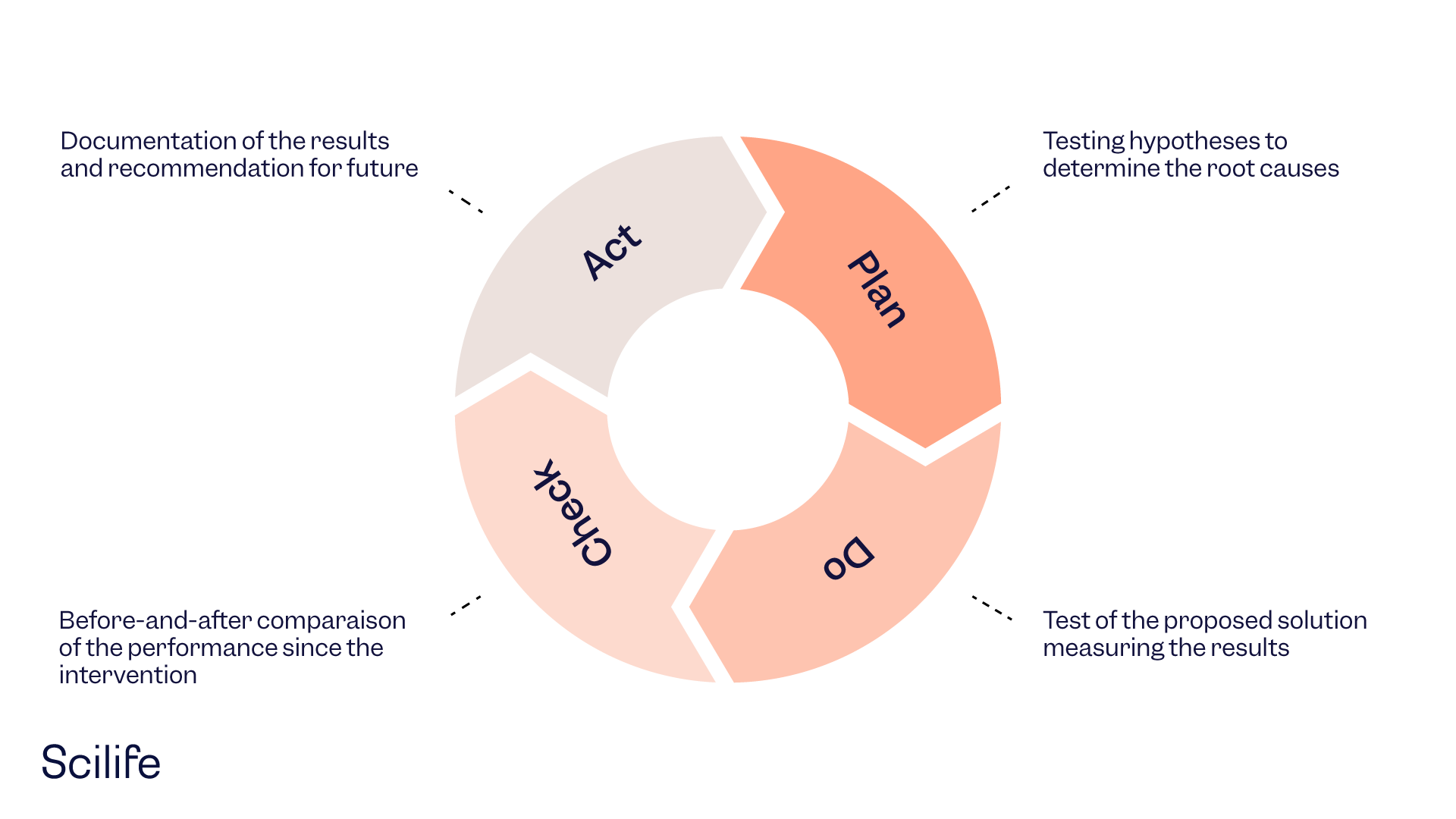
There are 5 steps to implementing a quality management system.
- In the first step, you identify your internal and external business context.
- In the second step, you plan what to do to deliver the product/service to the customer.
- In the third step, the do stage, you implement your operations plan.
- In the fourth step, the check stage, you assess the performance and identify which are the areas for improvement.
- Lastly, in the fifth step, the act stage, you analyze the defects and apply changes and education.
Let’s see one by one each step:
Step 1: The context
In this stage, your company needs to include an understanding of, and alignment with, the strategic direction of the company. The external and internal factors that may affect your business, your goals, and your objectives need to be clearly defined at this stage.
1. External context
To understand your external context, you need to consider several factors:
-
-
- the uncertainties and economic changes
- the market needs
- your competitors, suppliers, partners, and customers
- the regulatory bodies
- the changes in technology
- the social and cultural behaviors
- changes in the political landscape
- legal changes
- the economic environment
-
It is important to identify which are the interested parties who are affected by your work and understand their needs and expectations from you.
For example:
-
-
- According to ISO 9001, customers expect that an organization is able to consistently deliver products and services conforming to their requirements.
- According to Medical devices, patients expect that medical devices are safe, and will improve their health and even save their lives.
- According to Pharmaceuticals, patients expect that patients expect every dose of medicine they take is safe and effective, free of contamination and defects.
-
2. Internal context
To understand your internal context, consider issues related to company values, quality culture, knowledge, and current performance.
Once you have identified the organization and its context, and the interested parties and their needs, it is time to determine the scope of the QMS.
A QMS can be established using the PDCA cycle. It is an effective model to systematically manage, control, and continuously improve all processes, focusing on satisfying customer needs. Risk-based thinking is used to prevent risks and take advantage of opportunities.
Step 2: Plan
1. Define your quality policy and quality processes
In this stage, the management with executive responsibility takes accountability for the effectiveness of the QMS. The management demonstrates leadership and commitment by establishing quality intentions and directions to ensure they are understood by the whole company. It is necessary to receive feedback from employees to succeed. This is the quality policy supported by the strategic direction.
A quality policy defines the quality practices, resources, responsibilities, and activities that are relevant to the business. It also shows how the requirements for quality will be met according to the strategic direction.
Define clearly the quality of your product or service: specification limits and compliance requirements.
2. Strategic and tactical level: Define your business goals, objectives, and metrics
At this level, you refer to the plans and actions that your company is taking to achieve the vision and goals for the future according to the strategy.
Management defines the business goals, specific objectives, and metrics that will help to evaluate the performance.
It is important to define:
-
-
- What will be done
- What resources are needed
- Who is responsible for each process, task, etc
- When it will be completed
- How will the results be evaluated
-
Quality objectives should be aligned with the business goals and regulations.
The resources needed for the establishment, implementation, maintenance, and for continual improvement of a quality management system of quality are:
-
-
- People
- Infrastructure
- Process environment
- Monitoring and measurements
- Organizational knowledge
-
3. Operational level: Determine and map your processes
At this level, you need to Map your processes identifying the inputs and outputs, and the correlation and interaction between the processes.
A good way to do so is to document all the flows of the processes to assure they will be followed as defined.
It is time to apply risk management principles to identify the risks. Plan actions to address the risks by eliminating, preventing, or reducing the likelihood of happening.
What is a risk?
According to ISO 9001:2015: “The effect of uncertainty on an unexpected result”
-
-
-
- What could happen
- How likely it is to happen
- What effect it would have if it happened
-
-
Do also consider addressing opportunities. The new technology, digitalization, new practices, and quality culture can leverage the potential to gain competitiveness.
4. Determine your resources
Personnel
Map out your organizational structure and the key responsibilities of employees in every function. This will enable employees to understand what their key deliverables are in terms of quality, and how they can collaborate with employees in other functions.
Infrastructure and materials
The organization shall determine, provide and maintain the infrastructure and environment that is necessary for the operation of its processes and to achieve conformity of products and services.
Provide adequate facilities, environment, equipment, technology, tools, materials, hardware, and software, that are needed for your operations to obtain your product and services.
5. Define your quality system documentation: procedures, instructions, and records
Good documentation is key to operating in compliance with quality system needs and regulations requirements. The objective is to establish, control, monitor, and record all activities which directly or indirectly impact all aspects of the quality of the products and services.
Standard Operating Procedures, work instructions, and methods are used to give direction and indications, while records and reports are the proof that the processes have been done and give assurance of traceability.
Step 3: Do (Operations)
“Quality doesn’t happen by chance. Quality happens by design.”
In this stage, you execute your plans (what, who, when, how) for manufacturing activities. Use a system to monitor process performance, inspection, and testing activities, to verify that products and materials conform to specified requirements.
One of the ways of building the desired quality into the product is to ensure that the natural variation in the product is as low as possible and smaller than the defined quality specification. Control this variation. This is to ensure that the desired quality of the product will be manufactured consistently and repeatedly, batch after batch.
Step 4: Check. Monitor quality, analyze and review your process performance
“Without data, you’re just another person with an opinion” -
W.Edwards Deming
To ensure that a state of control is maintained and facilitates continual improvement, plan to monitor process performance and product quality, as well as the effectiveness of the quality management system.
This monitoring system should provide assurance of the continued capability of processes and controls to produce a product of desired quality and to identify areas for continual improvement.
The degree of customer satisfaction shall be monitored to determine their needs and expectations. You can use surveys, meetings, feedback, etc.
Analysis and evaluation of data:
-
- The conformity of products and services
- If nonconformances have been addressed
- The degree of customer satisfaction
- The performance of external suppliers
- If the actions to address risks have been effective
- The QMS performance
Retain appropriate documented information from monitoring as evidence of fitness for purpose.
1. Corrective action and preventive action (CAPA) system
Whenever there are complaints, product rejections, non-conformances, recalls, deviations, audit and regulatory inspections with findings, and trends from process performance and product quality monitoring, investigations should be done to find the root cause. The application of a CAPA methodology should result in product and process improvements and enhanced product and process understanding.
2. Change management system
Any change to be applied in any process, because of a CAPA, continual improvement, innovation, etc, needs to be evaluated and approved before implementation.
Changes must be done in a planned and systematic manner.
A change management system needs to be in place to ensure that the change is undertaken in a timely and effective manner without unintended consequences.
3. Management review of process performance and product quality
The management review helps to assure process performance and product quality are managed. It is useful to identify appropriate actions for improvement, training, operational changes, etc.
4. Periodic quality reviews:
It covers complaints, recalls, KPIs and metrics, customer satisfaction, etc.
5. Quality audits
It covers the results of self-audits, regulatory inspections and findings, external audits and other assessments, and commitments made to regulatory authorities.
Internal audits shall be conducted at planned intervals to provide information about the conformity of each process to the QMS requirements and regulations.
Step 5: Act. Define your defects and take action to improve
1. Identify your defects and areas for improvement
It is the most important part of the Quality Management System.
-
-
- Address non-conformities and deviations
- Focus on root-cause investigations
- Apply CAPAs
- Use lessons learned
- Anticipate and react to risks and opportunities
- Enhance drive for innovation
-
Without actions for improvement, the efforts and activities applied throughout the system will be fruitless.
The actions for improvement drive your system to be better. Use nonconformities as an opportunity for improvement.
It potentially engages your customer, reduces error, and saves money.
This is why we continually need to do things smarter and better.
The ways to be effective can be summarized in 4 manners:
-
-
- Being reactive to non-conformities
- Being gradual, looking for incremental improvements
- Being creative, using innovation to drive improvement
- Being transformational, implementing big changes, such as digitalization
-
2. Define organization knowledge and trainings
Determine which is the knowledge that is necessary for the operations and processes functioning. Document the knowledge of procedures and work instructions in a clear and understandable way. Share the knowledge with employees and plan a learning path to upgrade people’s skills according to business objectives and plans. Give appropriate education and training to ensure that people are competent in their work. Evaluate the effectiveness of training plans and apply actions if needed.
Curious to know how a Smart Quality Platform is different from a QMS? Discover more about it here.