
This is a story of triumph over tragedy. A story shaped by pivotal events that stress the importance of regulatory oversight in the production of pharmaceuticals and other medical products. This is the story of how the Good Manufacturing Practices (GMP) within the GxP (Good Practices) industry came to be.
Join me on this journey of tragedy, regulations, and patient safety, as we delve into the historical origins and evolution of GMP, starting with the horrors of diphtheria in the early 1900s and tracing its development through many more health incidents such as the Biologics Control Act of 1902, the impact of Upton Sinclair‘s ‘The Jungle‘ in 1905, and the transformation of the US Bureau of Chemistry into the full-fledged Food and Drug Administration (FDA) in 1930. I will also discuss key incidents like the elixir sulfanilamide tragedy in 1937, the sulfathiazole disaster in 1941, and the thalidomide disaster in 1962.
My goal throughout this trip to the past is to highlight the critical role these events have played in shaping today’s regulations, standards, and GMP, all in the context of patient safety and innovation. Ultimately, the tragic events mentioned here have helped raise public awareness of the dangers associated with inadequate Quality Control and laboratory testing measures; and as horrid as they were, these incidents have led to the improved safety standards and regulations we now have in the Life Sciences.
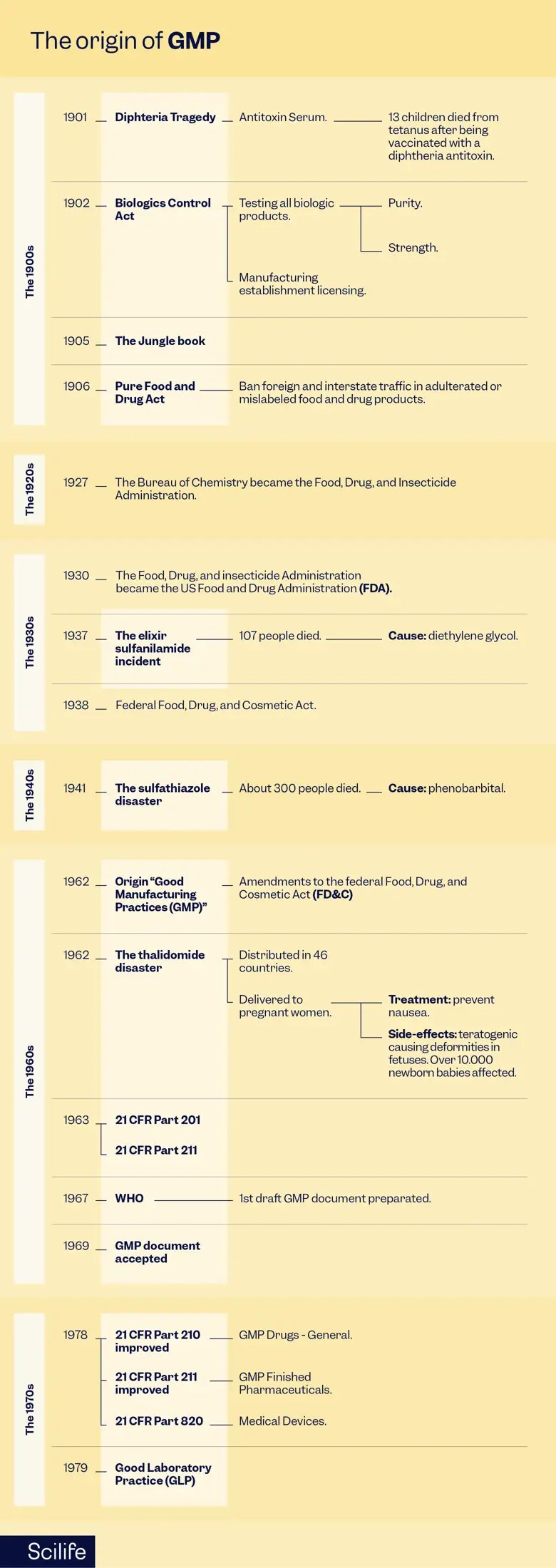
Early 1900s – The Diphtheria Tragedy
At the beginning of the 20th century, the healthcare situation in the United States was marked by high infant mortality rates and widespread diseases such as tuberculosis and diphtheria. Diphtheria, a deadly bacterial infection, was a significant threat to public health.
The first glimmer of hope came with the development of an antitoxin serum that promised to treat diphtheria. This serum was derived from horses that had been inoculated with small amounts of diphtheria toxin. The serum was then purified to isolate the antitoxins, which proved effective in halting the progression of the disease. However, the product was manufactured in local establishments with no harmonized controls.
Unfortunately, in 1901, a devastating incident occurred in St. Louis, Missouri, where 13 children lost their lives due to a tetanus infection after receiving a diphtheria antitoxin. It was later discovered that the serum they had all used was derived from a horse with obvious signs of tetanus. Due to this tragedy, it became apparent that there were no uniform controls in serum production, and that the final product was not adequately tested or inspected.
1902 – The Biologics Control Act
In response to the diphtheria tragedy, the US government introduced the Biologics Control Act of 1902, also called the Virus-Toxin Law. This landmark legislation aimed to regulate biological products, including vaccines, serums, and antitoxins. It mandated the testing of these products for purity and potency and required licensing for all establishments involved in their manufacture or sale. This act laid the foundation for future regulations, emphasizing the importance of high-Quality raw materials in medicinal product production.
1930 – The Transformation into the FDA
In 1930, the Bureau of Chemistry, which had regulated foods and drugs since 1906, underwent a transformation and officially became the FDA. This new government body was given the power to inspect food, drug, and cosmetic manufacturers. It could also require these manufacturers to provide evidence of the safety of their products before they were allowed to sell them. This act was a major milestone in consumer protection and product safety.
1937 – The Elixir Sulfanilamide Incident
The elixir sulfanilamide incident of 1937 stands as a stark reminder of the dire consequences of inadequate regulation. Diethylene glycol was used as a solvent by a Tennessee pharmaceutical company to manufacture sulfanilamide, a drug for the treatment of streptococcal infections. There were no clinical trial regulations in place at the time, but the company released the product without rigorous testing anyway.
Tragically, 107 people, including many children, lost their lives after consuming the elixir. The diethylene glycol in the formulation was found to be poisonous. In response to this horrific incident, the US congress passed the Federal Food, Drug, and Cosmetic Act of 1938. This pivotal legislation mandated safety testing for all products before marketing and extended the FDA’s oversight to cosmetics and therapeutic devices. It marked a significant turning point in pharmaceutical regulation and emphasized the importance of rigorous testing and Quality Control.
1941 – The Sulfathiazole Contamination
In 1941, another devastating incident occurred when approximately 300 people died after taking sulfathiazole tablets manufactured by the New York-based Winthrop Chemical Company. An FDA investigation revealed that the tablets were contaminated with phenobarbital. This incident prompted the FDA to revise its manufacturing and Quality Control requirements, setting the stage for the development of what would later become GMP.
1962 – The Thalidomide Deformities
The thalidomide disaster of 1962 stands as one of the most tragic events in GMP history. Thalidomide, initially introduced as a mild sleeping pill, was soon prescribed to pregnant women to help alleviate their morning sickness. Unfortunately, thalidomide is teratogenic, causing severe limb deformities in thousands of newborns worldwide.
In the wake of this catastrophe, regulators recognized the urgent need for improved drug testing protocols. Hence, the first official GMP guidelines for the processing, packing, and holding of finished pharmaceuticals were introduced in 1963. These regulations emphasized the importance of safety and efficacy testing, marking the beginning of modern clinical trials regulations.
Conclusion
The historical journey of GMP within the GxP industry is a testament to the essential role of regulations in ensuring patient safety and fostering innovation in the Life Sciences. From the diphtheria tragedy of the early 1900s to the thalidomide disaster of 1962, each incident served as a catalyst for regulatory reform and the development of GMP standards. These regulations, rooted in lessons learned from past tragedies, have continued to evolve and adapt, ultimately leading to the establishment of current Good Manufacturing Practices (cGMP).
As I reflect on this historical progression, it is evident that the pursuit of patient safety and product Quality has been at the heart of GMP’s evolution. Today, GMP standards are integral to the pharmaceutical and healthcare industries, ensuring that medicines and medical products meet the highest standards of safety, efficacy, and Quality. Through the lens of this historic review, we are reminded of the vital importance of regulatory oversight and the ongoing commitment to safeguarding public health.
If you want to know more about Good Manufacturing Practices in the European Union, check the following video:
References
FDA History Timeline
The official timeline provided by the FDA offers a comprehensive overview of key events and milestones in the FDA’s history, including its transformation from the Bureau of Chemistry and the impact of various regulatory acts.
Biologics Control Act of 1902
Information about the Biologics Control Act of 1902 can be found on the website of the FDA. This source provides historical context for the act and its significance.
Pure Food and Drug Act
The FDA’s page on the Pure Food and Drug Act provides insights into how Upton Sinclair’s ‘The Jungle’ influenced the passage of this landmark legislation.
Elixir Sulfanilamide Incident
The FDA’s page on the Elixir Sulfanilamide incident offers a detailed account of the tragedy, its aftermath, and the resulting regulations.
FDA Regulations
The official FDA website provides a wealth of information on regulations, including GMP, and the historical context in which they were developed.
cGMP Overview
These additional sources can further substantiate the historical events and regulatory developments discussed in the blog post, offering readers authoritative references for a deeper understanding of the topic.





