
With the European Medical Device Regulation (EU MDR, 2017/745), the Unique Device Identifier (UDI) became mandatory for medical device manufacturers in the European Union. The MDR aligned the EU with the US market, where the UDI system had already been implemented since 2013 by the Food and Drug Administration (FDA).
What is the Unique Identifier (UDI)?
The UDI is a code affixed to medical devices to identify and track them through the supply and market chain. The medical device manufacturer typically generates the code, but it can also be generated by an economic operator on authorization from the manufacturer.
In 2007, the FDA was requested to establish regulations that reduced device errors affecting patients and allowed for the identification and recall of defective devices much sooner. The FDA came up with the idea of a national tracking system and began running pilot programs to develop it. The pilot programs included universal product codes as mandatory parts of reimbursement claims in health insurance cases, saving more than $30 million in supply contracting. Likewise, data showed that defective devices were identified far quicker than previously, and supply chain efficiency soared.
Following the success of the pilot programs, the FDA published the final UDI rule in 2013.
Also in 2013, the International Medical Device Regulators Forum (IMDRF) published guidance on UDIs of medical devices and set the stage for a globally harmonized UDI system.
Due to local differences in regulations and guidelines for the UDI, making it complicated to collect and maintain data across markets, a globally harmonized UDI system still needs to exist. Some forms of UDI systems exist in South Korea, The Netherlands, United Arab Emirates, China, Taiwan, Saudi Arabia, Australia, Brazil, Canada, Japan, Singapore, and the UK.
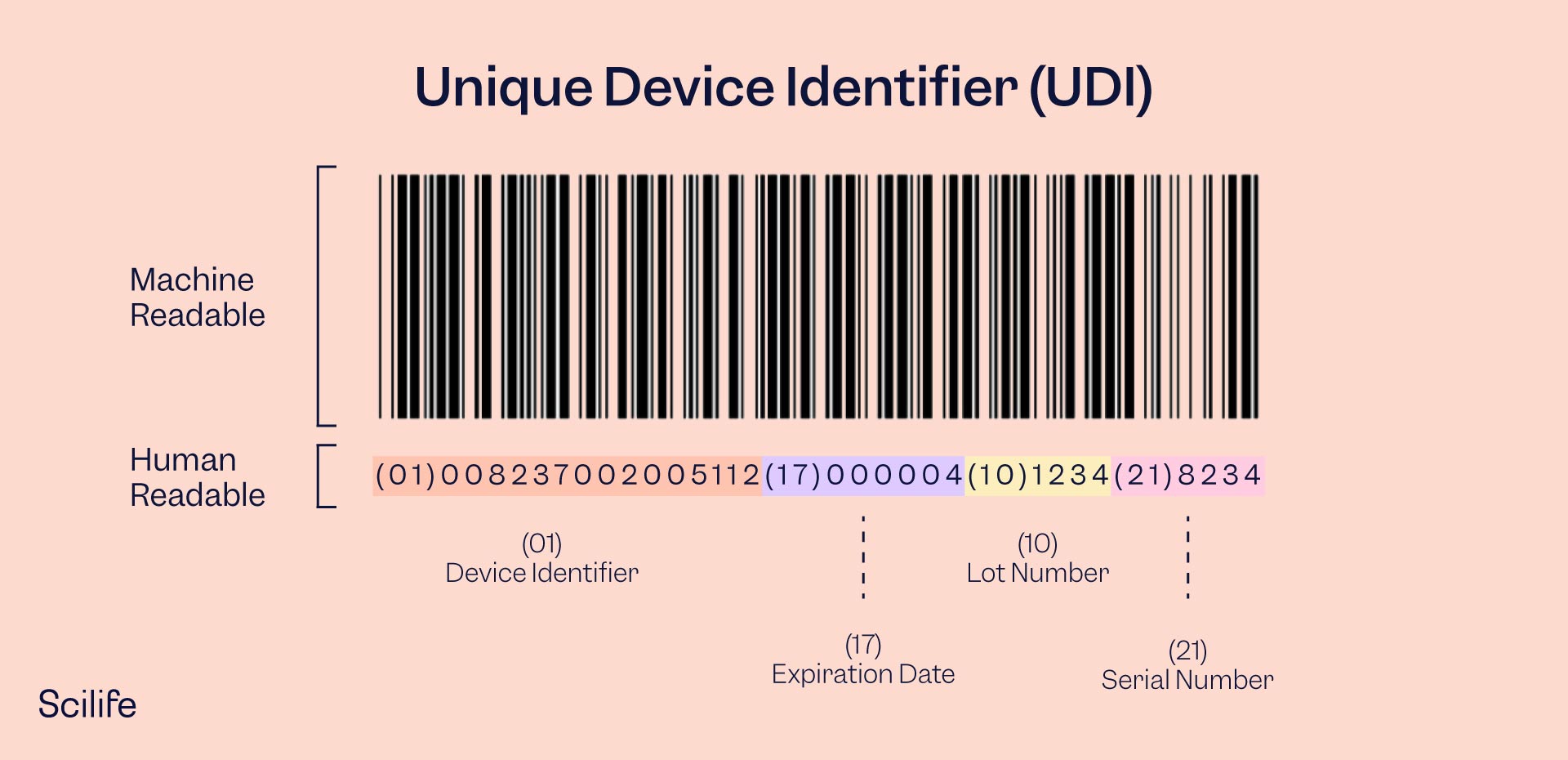
The UDI consists of a Device Identifier (DI) and a Production Identifier (PI).
The Device Identifier is device specific and provides information on the manufacturer, authorized representative, device model, and further device information. The DI should always be the same as it pertains to static information and does not change for every device manufactured.
The Production Identifier (PI) identifies the variable characteristics of production, such as manufacturing and expiration date, lot numbers, serial numbers, etc. The PI changes from device to device according to when and how the device was manufactured.
The Device Identifier and Production Identifier comprise the full UDI, with the DI first and the PI second.
What products require UDIs?
In the EU, all medical devices except custom-made and performance/study investigational devices require implementing the UDI system.
In the US, a few devices are exempt from the UDI requirement, such as Class I devices that bear the Universal Product Code (UPS), devices destined only for export from the US, and custom-made devices, among others.
Who issues UDIs?
Each health authority can create its own list of UDI issuing entities. In the EU and US, the following entities are authorized to issue UDIs:
GS1
GS1 is a nonprofit standards agency that sets standards for various processes, such as supply chains, data exchange, and healthcare. The Universal Product Code (UPC) is a GS1 standard. Most medical device manufacturers use GS1 for their UDI designation.
HIBCC
The Health Industry Business Communications Council is a nonprofit organization that sets standards for the healthcare industry.
ICCBA
The International Council for Commonality in Blood Banking Automation is a non-governmental organization focusing on standards for human-origin medical products. The ISBT 128 standard is implemented in more than 75 standards by ICCBBA. Only products of human origin are designated by the ICCBBA standard.
Informationsstelle für Arzneispezialitäten (IFA) GmbH
IFA GmbH assigns a Pharmacy Product Number (PPN) as part of their coding under the UDI system. The PPN system is mainly used for pharmaceutical products but can also be used for medical devices.
Why do we need the UDI system?
The UDI system is implemented to ensure patient safety by increasing device traceability. When a device can be correctly traced, it allows for accurate adverse event reporting and field safety corrective action implementation. Adverse events and trends are discovered quicker, and manufacturers can respond faster.
The benefits to the UDI system include better device procurement and management in healthcare systems, improved traceability, better protection against counterfeit or false devices, enhanced post-market surveillance, earlier detection of any potential safety issues, better recall management, increased public access to device information, more accurate cooperation between regulatory authorities and databases in case of adverse events, and improved management and analysis of field safety corrective actions, among others.
Where should the UDI be placed on the device?
Your UDI should be placed on the product and packaging labels and, in some instances, placed on the device itself.
The UDI should be presented in two formats:
Automatic Identification and Data Capture (AIDC)
Typically, a one- or two-dimensional barcode can be quickly scanned with a barcode reader. This format can also be entered into electronic patient records or digital systems for easy device identification.
Human Readable Interpretation (HRI)
A format that humans can read without the use of technology, i.e., standard text or numbers.
In the case of reusable devices, the UDI should be included directly on the product, for example, via laser etching, because the packaging is discarded after first use.
If there isn’t room on the primary packaging, which is the case for many small IVDs, the UDI can be placed on the following packaging level. If there is only space for one of the UDI formats, the AIDC takes precedence, except in cases where devices are intended for use outside of healthcare facilities, in which case the HRI format takes precedence.
You can see more UDI placement requirements in Annex VI Part C of the MDR and IVDR.
Are UDIs public?
Generally, yes.
UDI’s must be submitted to the health authority’s Unique Device Identification Database (UDID). In the EU, it should be submitted to the European Database on Medical Devices (EUDAMED) and to the Global Unique Device Identification Database (GUDID) in the US. The submission should be made before placing the device on the market.
Once the UDI is submitted to the database, the health authority typically provides the content to the public as a searchable database/index.
How do I create a UDI for my medical device?
Creating and managing a UDI for each medical device in your portfolio can be complex and time-consuming. The Udi process should be carefully planned to avoid too many road bumps.
The process can be described in 5 steps:
1. Create a UDI plan and team to ensure you reach the correct deadlines, your systems are ready, and the exact devices requiring UDI are identified appropriately.
2. Choosing an issuing agency that works for your company and portfolio.
3. Collect the required data.
4. Make sure your data is clean, up-to-date, and complete. This might be the hardest step, as every data point must be analyzed and harmonized.
5. Submit the UDI to the authorities and affix it to your products. Don’t forget to continuously update and maintain your UDI records and labels as necessary.


