Every year, the FDA issues hundreds of warning letters along with inspections of manufacturing facilities. You should take the warning letters seriously because they act as a precursor to a worse failure. You should always take proper corrective and preventative actions accordingly and make a comprehensive gap analysis based on the mentioned requirements to be fully compliant.
We’ll focus on the most cited requirements in the FDA’s warning letters received by the drug manufacturers. We'll also share the main reasons behind the warning letters that drug manufacturers received in 2022.
Worst 14 FDA Warning Letters Citations for the Fiscal Year 2022
During the fiscal year 2022, 152 compliance actions were issued based on FDA Drug warning letters worldwide, and 72 % of them were from domestic sources.
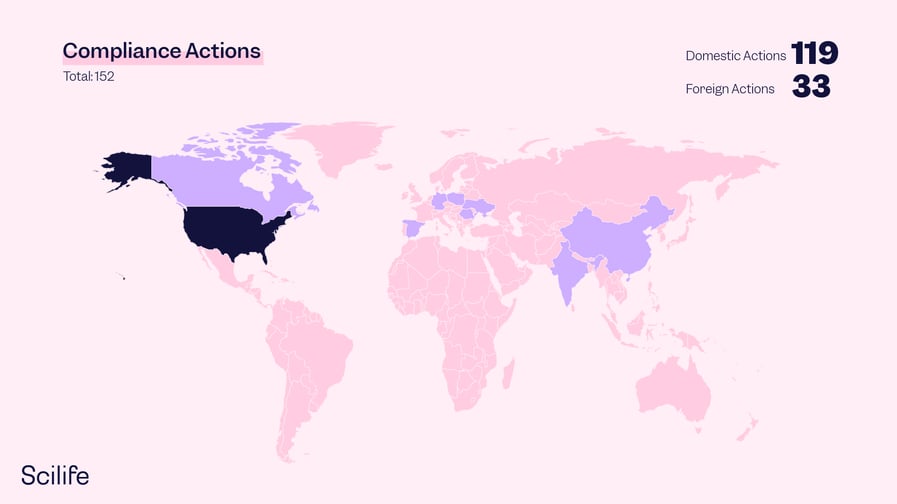
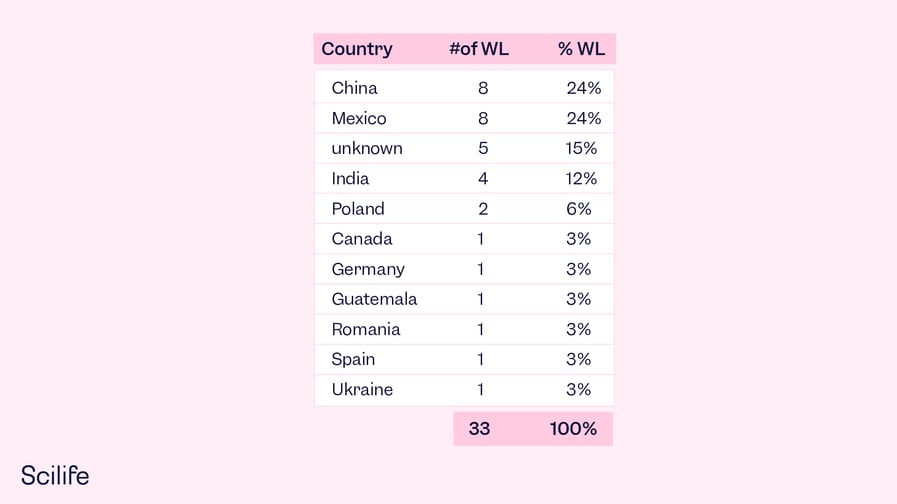
On the other hand, several countries manufacture and market their drugs in the US market. That´s 38% of all warning letters. Which countries have the most FDA drug warning letters in 2022? In 2022, it was China and Mexico who shared the joint highest number.
For further info regarding the FDA´s complete use of data, check their Dashboard Page here.
Look closely at FDA Drug Warning Letters sent to pharmaceutical manufacturers in the fiscal year 2022. We gathered all of them to explicitly describe the GMP deficiencies in relation to the paragraphs of 21 CFR 211. And we put together all citations to show you the most cited GMP requirements in the FDA’s drug warning letters…
Worst 14 Drug Warning Letters
|
CFR Clause |
CFR Clause |
# of Warning Letters citations |
% of WL citations |
|---|---|---|---|
|
Responsibilities of quality control unit. |
33 |
22,1% |
|
|
Consultants |
33 |
22,1% |
|
|
Written procedures; deviations. |
29 |
19,5% |
|
|
Production record review. |
26 |
17,4% |
|
|
Meaning of “intended uses” |
25 |
16,8% |
|
|
Testing and release for distribution. |
23 |
15,4% |
|
|
Over-the-Counter (OTC) Human Drugs Which Are Generally Recognized as Safe and Effective and Not Misbranded |
23 |
15,4% |
|
|
Testing and approval or rejection of components, drug product containers, and closures. |
21 |
14,1% |
|
|
Eligibility for classification as generally recognized as safe (GRAS). |
20 |
13,4% |
|
|
Equipment cleaning and maintenance. |
18 |
12,1% |
|
|
General requirements. |
15 |
10,1% |
|
|
Format and content requirements for over-the-counter (OTC) drug product labeling. |
12 |
8,1% |
|
|
Stability testing. |
11 |
7,4% |
|
|
Automatic, mechanical, and electronic equipment. |
11 |
7,4% |
The main reasons behind the drug warning letters
The most cited CFR requirement is 211.22 - Responsibilities of the quality control unit - Subpart B - Organization and Personnel. This explains the responsibility of the Quality assurance or Quality unit. Unfortunately, most drug manufacturers who received the warning letter failed to take responsibility as they should have regarding the FDA’s cGMP requirements. 33 out of 149 Drug Warning letters cited this requirement, but what are the violations in detail regarding 211.22? Now, we’ll dive deeper into this requirement to have a better understanding of what are the most failed areas.

How to tackle most common drug cGMP failures
We addressed top drug citations and offered insights so that you consider them proactively to ensure compliance. If you feel you need an expert or more resources to achieve or remediate your quality management process according to the most cited FDA CFR.211 requirements, Scilife offers you numerous sources to tackle. Here are the most valuable blog posts and downloadables:
FDA Drug Warning Letter trends
Warning letters and inspections have correlations. We will look into the similarity and differences between them by comparing the FY 2022 warning letters with the inspections of the past five years…
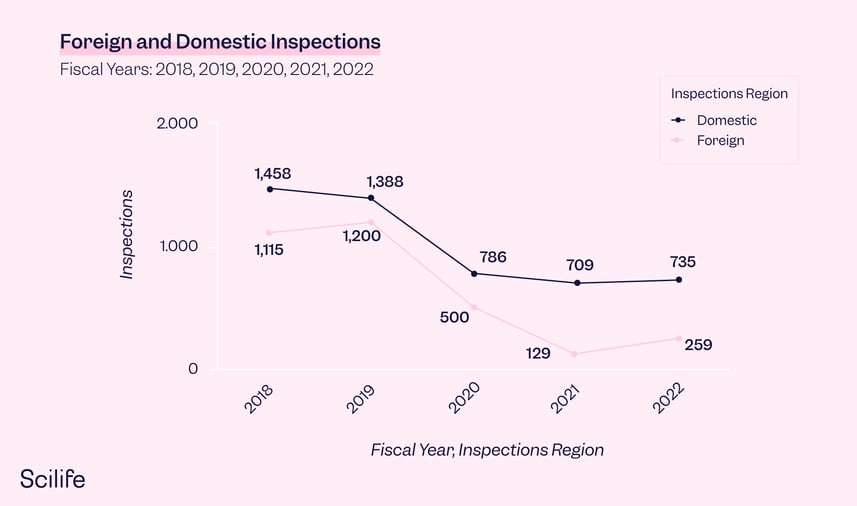
1. The Fiscal year 2020 saw the most warning letters issued to drug manufacturers in five years
Looking at the two charts together, we see that drug warning letters reached the highest number in past years. But on the other hand, the total number of inspections decreased significantly in 2020, and has continued to fall each year.
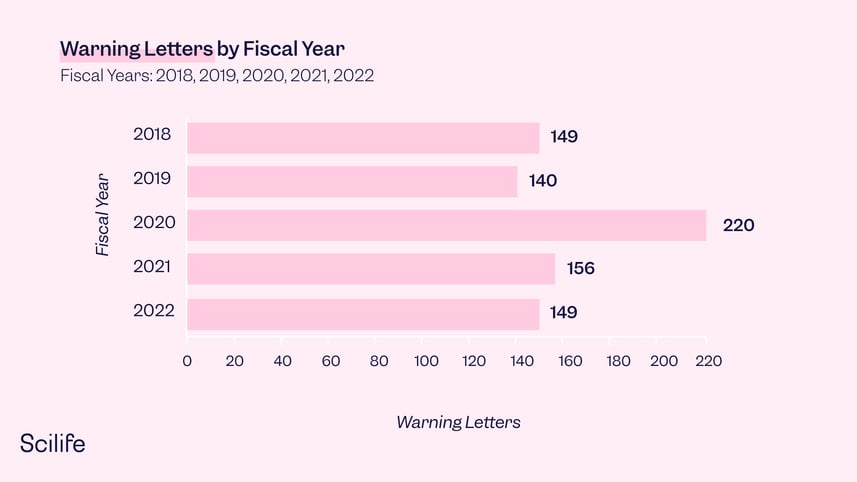
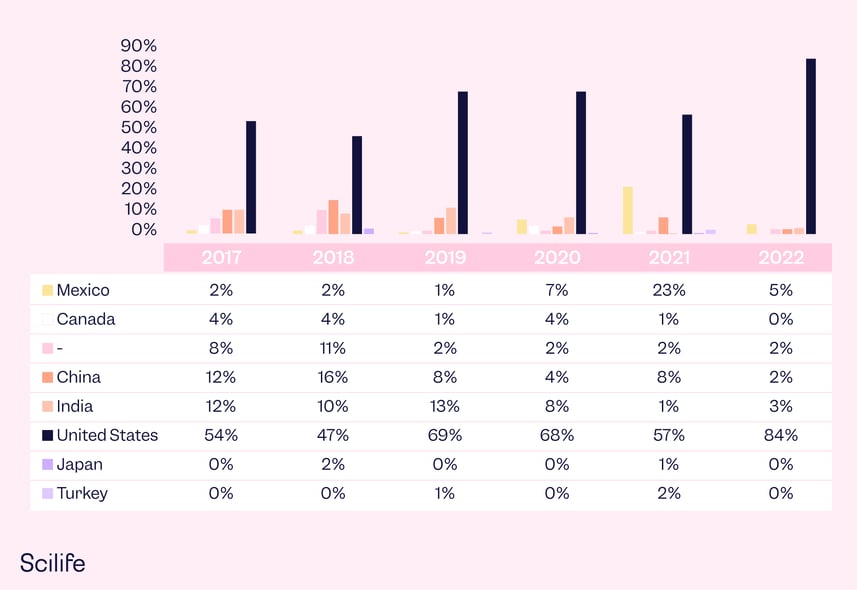
2. Distribution of pharmaceutical manufacturers who received drug warning letters
In 2022, At least 85% of drug warning letters were issued to companies located in the United States, which has the majority of the warning letters over the past 5 years. A detailed list of each region broken down by past five years is below:
3. Trends of inspection observations for drugmakers
There is a significant similarity in citations between inspections and warning letters. We’ve reviewed citations of FY2022 FDA Drug warning letters. As you’ve seen above, 4 out of the top 5 citations are the same! This is very important for all manufacturers, not only those who received these letters or were inspected by the FDA. So here is your takeaway: review your processes in light of those citations!

Lessons to learn
Warning letters and inspection observation data are potent resources for understanding regulatory focus areas and benchmarking potential weaknesses within the quality system.
There has been a continued focus on investigating and citing companies for violations of basic GMP regulations, along with an overall increase in total citations across the pharmaceutical industry.
So, it is vital to heed warning letters from the FDA. And by taking measures to avoid them in the first place, you won't just be fully compliant, you´ll be saving costs and preventing possible failure.
Did you know that a Smart Quality Platform can help you avoid receiving a warning letter? Discover why!