Most pharma and medical device companies are increasingly adopting electronic Quality Management Systems (eQMS) to streamline their quality management processes. A well-designed quality management system helps you to meet customer satisfaction and regulatory compliance. It also fosters a continuous improvement culture. However, despite all the benefits and the undeniable truth that an electronic system facilitates better QMS, implementing an eQMS is certainly not the end of your project - but rather, the beginning of a journey.
The majority of people don’t realize that you will be managing all the documentation, training, changes, audits, and suppliers within the eQMS. Your processes may need to be adjusted to comply with regulatory requirements and internal quality standards. And what forms the basis of those quality sub-processes? More documents, of course!
Today, we’ll explore the five must-have templates you need for managing your eQMS in more detail, along with some tips for writing them.
What is essential QMS documentation?
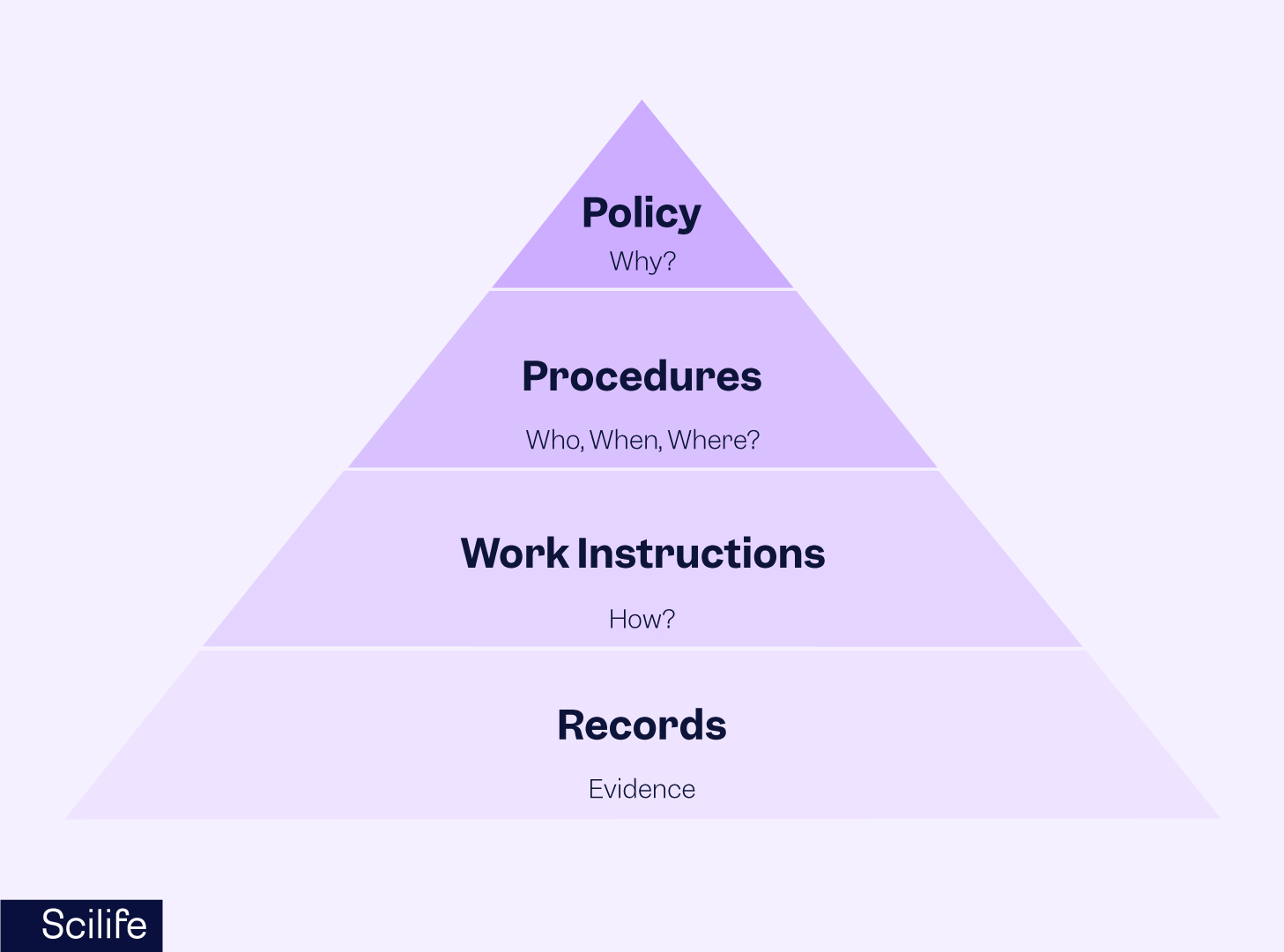
Documentation is an integral part of the quality management system that ensures consistency in processes and procedures. The documentation hierarchy forms the QMS documentation structure by containing different types of documents within the QMS. It makes it easy to understand, communicate, and visualize the documentation structure and organize documents in a meaningful way. It also provides evidence for the objectives and goals.
Each documentation level in the pyramid builds upon the previous one and contributes to the overall effectiveness of the QMS. ISO 9001:2015 does not require a document hierarchy, so you may define your documentation structure. The QMS documentation hierarchy presented below can be redesigned and filtered for the intended use of the documented information.
-
Quality Manual and Policy
-
Procedures (SOPs)
-
Work instructions (WIs), Forms, Templates
-
Retained documents (i.e. records)
What are the key QMS documentation requirements for pharmaceutical and medical device companies?
QMS documentation requirements vary depending on the industry. Let’s look at the key QMS requirements for pharma and medical device companies.
ISO 9001:2015
This standard is the most known and most implemented QMS standard for quality management systems. As it requires companies to maintain and retain documented information to support their processes and ensure they are executed as planned, many pharmaceutical companies follow this standard. Examples of QMS documentation required by ISO 9001:2015 include:
-
- Quality Policy
- Documented Procedures
- Compliance Records
- Internal and External Audit Trails
- Records of Non-Conformities and Corrective Actions
- Risk and Opportunity Management Records
- Staff Training and Competency Records
- Records of Quality Plans and Objectives
ISO 13485:2016
ISO 13485:2016 requires companies to create and manage documents and records to ensure effective quality processes planning, operation, and control. Examples of QMS documentation include:
-
- Quality manuals
- Medical device files
- Training records
- Complaint handling records
FDA 21 CFR Part 211 (cGMP)
The FDA’s cGMP requires written procedures, records, and reports to be managed and kept readily available for inspection during the retention period. It requires pharma companies to have QMS documentation, including batch production records, equipment use logs, complaint files, and sanitation procedures, among others.
FDA 21 CFR Part 820 (QSR) or newly named QMSR
The FDA 21 CFR 820 requires manufacturers to establish and maintain procedures to control all documents related to medical devices, including the creation, review, approval, and change processes such as design history files (DHRs), device master records (DMRs), nonconformance documents, equipment calibration records, CAPA documents, and more.
ICH Q10
The ICH Q10 guideline outlines the requirements for the quality manual, quality policy, CAPA documents, product discontinuation reports, deviation documentation, and more.
What are those 5 must-have QMS templates?
Since many regulations require pharma and medical device companies to have thorough QMS documentation, it is vital to have templates for the most known and most used quality document types. It’s not sustainable to manage an eQMS without standardized templates. Once you have completed the implementation of your eQMS, you should look for improvements. We recommend you start with having predefined document templates, which could make a massive difference.
Now, let’s explore the five must-have templates that Scilife has created for you that may be needed for managing your eQMS.
1. Quality Manual Template
The top-level document of a quality management system is the quality manual. It explains how QMS functions and acts as a guide on how to maintain the quality system. The document structure and content can vary depending on the organization’s size, the complexity of its operations, and the competency of the personnel. A quality manual template can help elevate your organization's quality management system. Typically, the quality manual includes the following elements:
-
- Cover page: Document Control (e.g. version history, and approval)
- Title and table of contents
- Scope
- Normative References
- Terms and Definitions
- Quality Management System
- Management Responsibility
- Resource Management
- Product Realization
- Measurement Analysis and Improvement
- References
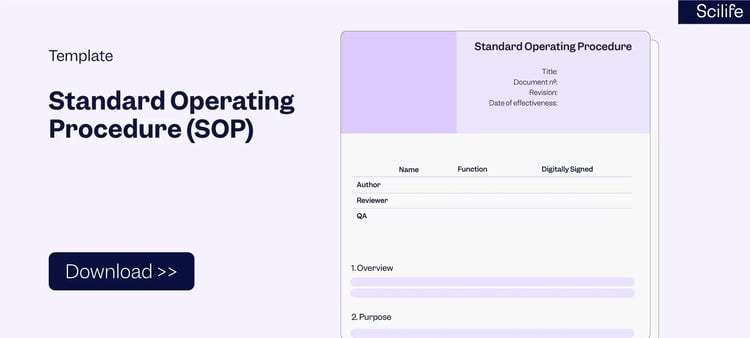
2. SOP Template
SOP is a documented guide that provides step-by-step instructions and activities to be followed in the performance of specific processes within a Quality Management System. SOPs should be robust and serve as the backbones of your Quality Management System. The SOP structure does not differ much but it can vary depending on the complexity of the process. However, a well-designed SOP template can help you to elevate your processes. Typically, a SOP includes the following main elements:
-
- Cover page: Document Control (e.g. version history, and approval)
- Title and table of contents
- Overview
- Purpose
- Scope
- Terms and Definitions
- Responsibilities
- Procedure
- References
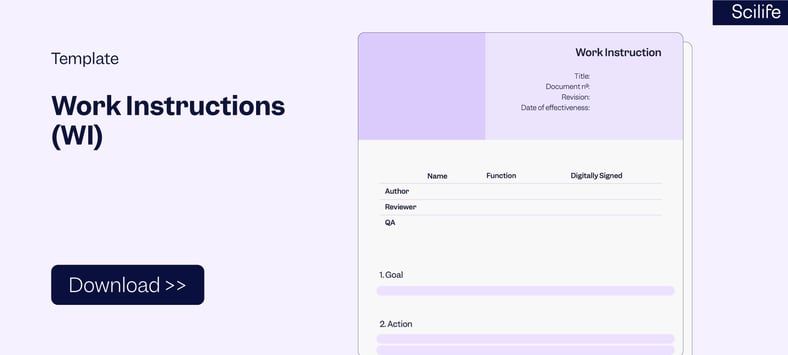
3. Work Instructions Template
A work instruction (WI) is a more detailed instructional document that outlines specific operations associated with a role and steps of a subprocess. It streamlines processes for optimal efficiency. The WIs help to ensure tasks are carried out consistently and effectively and meet the applicable requirements of the quality management system.
Some processes can be complex. They need further instructions to be able to capture each detail and explain everything properly. Additionally, documents, especially the more complex ones should be well-crafted according to details and the overall structure. The final document and instructions should be easy to read and digest by employees. Therefore, you should cut the process into smaller pieces of documents that we call: Work Instructions.
Generally, work instructions have the same structure as procedures and cover the same elements; however, work instructions include details of activities that need to be realized, focusing on the sequence of steps, tools, and methods to be used and the accuracy required. It is possible to reduce the need for extensive work instructions by training personnel and using competent personnel.
Typically, a Work Instruction Template includes the following main elements:
-
- Cover page: Document Control (e.g. version history, and approval)
- Title and table of contents
- Purpose / Goal
- Scope
- Terms and Definitions
- Responsibilities
- Procedure / Checklist / How-to guide / Step-by-step instructions
- References
- Related Documents
- Attachments
4. CSV Template
CSV (Computer System Validation) has detailed documentation. The CSV documents should outline steps for someone reading it to repeat the process. Therefore, it is critical to use clear and concise language and a template to cover all activities.
Before any computer system is used, documents will outline specifics such as:
-
- Define the purpose of the computer system in question
- The features it needs
- The hardware it needs
- When it is used
- The requirements it must meet
- The defined specifications and used throughout the CSV process – continuing throughout the computer system's life.
This ready-to-use template will help you stay in charge of all the tasks related to Computer System Validation deliverables, following GAMP 5. So forget about wasting hours on verification and data mapping. Instead, you can use our comprehensive template to validate any software and ensure it meets all your usage and regulatory requirements.
Typically, a CSV Template includes the following main elements:
-
- Cover page: Document Control (e.g. version history, and approval)
- User Requirement Specification (URS)
- Risk Assessment (RA)
- Functional Requirement Specifications (FRS)
- Configurational Requirement Specifications (CRS)
- PQT
- Traceability Matrix (TM)
- Risk Assessment (RA) Matrix
5. Root Cause Analysis Template
Root cause analysis methods help you find the root cause of a problem and identify possible solutions by reviewing the underlying issues.
To improve your risk management strategy, you can use several risk assessment tools and templates. This helps you uncover the underlying chain of events (or causes) that result in non-conformities.
Typically, a Root Cause Analysis Template includes the following main elements:
-
- Cover page: Document Control (e.g. version history, and Opening and Closure approvals)
- Issue Details
- ID / Title / Name
- Date issue reported
- Issue to report
- Issue description
- Source(s)
- Criticality Level (Low / Medium / High)
- Possible Root Cause
- Cause description
- Probability Level (Low / Medium / High)
- Details
- Suggested Solutions
- Solution description
- Risks
- Measurement of Success
- Attachments
If you like our templates, find other resources that we have prepared for you!
Best Practices for QMS Documentation using well-structured template
We have discussed the five must-have documents for managing your QMS. Now let’s learn some tips for writing and developing these documents.
- Use a distinct structure
The documentation structure must be clear and logical so that information can be easily found. To navigate between different documents in QMS documentation, the structure must be consistent.
- Keep it simple.
The documents should not only be used for daily reference, but also for teaching newly hired employees. Write each document as if you were explaining it to someone unfamiliar with the subject. The language in QMS documentation should be clear, concise, and easily understandable.
- Avoid technical jargon or complex terminology
This may impact comprehension. Aim for simplicity to improve clarity and promote effective communication. Or define all the terminology in the Terms and Definitions section of the document.
- Add references as many as needed
If a process or activity is already well explained in another SOP, there’s no need to duplicate the content. You can address where it’s written.
- Define a responsibility assignment matrix.
The team roles and responsibilities must be clear at any stage of the process and to any actor involved.
- Stay current.
Processes, people, and their roles can change—basically, anything can change. This is why the SOP and other document content must align with the current situation at your company.
- Make sure your documentation is fit for purpose
The QMS documentation should be tailored to the specific needs of the company and its processes, as well as to the intended user’s needs.
- Use visuals
Visuals can be included to make QMS documentation easier to understand. Companies can incorporate visual aids such as flowcharts, diagrams, and tables to convey complex information efficiently.
Scilife is a fully validated eQMS software solution for life sciences designed to simplify and optimize quality documentation management, offering a range of features and benefits.
Scilife uses highly customizable documentation templates for document creation. You can use our complementary template package or create your own templates.
Final Thoughts
Scilife offers a smart QMS software solution with robust quality management capabilities tailored to life science companies. We provide Quality Document Management System Software that streamlines QMS documentation management. Furthermore, you can leverage your eQMS by using document templates that can save you a great deal of time and resources.